|
||||||||||
Date: September 24, 2024
by Chaya Venkat
Related Articles:
Editor's Note: Subsequent to the publication of this article, an editorial appeared in the Journal of Clinical Oncology addressing many of the issues raised here. The editorial, authored by Drs. John C. Byrd and Joseph Flynn of Ohio State, asks if we have forgotten the purpose of phase III clinical trials, specifically in context of the approval of Campath as first line therapy based on the results of the CAM307 trial discussed above. The editorial may be accessed free of charge through the following link: Byrd Editorial in JCO.

On September 19, 2024, the U.S. Food and Drug Administration expanded labeling and granted regular approval for single-agent Campath (Genzyme Corporation) as frontline therapy for the treatment CLL. Campath was initially approved in 2024 but only for relapsed cases, or in combination with other drugs. This is an important hurdle for Campath (also known as alemtuzumab). It is now the only monoclonal antibody that has been approved by the FDA for use as single agent and in previously untreated CLL patients.
However, in the opinion of this interested and strictly layperson observer, it is worth taking a second look at this development, notwithstanding the glowing press releases from the company's marketing machine. The million dollar question is this: now that we have access to this drug as frontline therapy, is this the slam dunk choice for untreated patients? Just because you couldda, does that mean you shouldda, wouldda?
This study compared the efficacy of Campath versus chlorambucil (why chlorambucil? Read on!) as frontline therapy for CLL. The initial results were published at ASH 2024 as abstracts (below). I am still trying to find the full length peer-reviewed article, but have not had much luck thus far on that score. I will keep looking. Here is my cheat-sheet version of the trial and its results:
| FISH Abnormality | Campath | Chlorambucil | ||||
| N | ORR (CR) | PFS months | N | ORR (CR) | PFS months | |
| 13q Only | 33 | 91% | 21.5 | 34 | 62% | 13.1 |
| 11q | 23 | 83% | 8.5 | 31 | 32% | 8.6 |
| 17p | 11 | 64% | 10.7 | 10 | 20% | 2.2 |
| Trisomy 12 | 27 | 85% | 18.3 | 12 | 75% | 12.9 |
| "Normal" | 25 |
80% | 20.7 | 26 | 69% | 14.3 |
| Full Cohort | 149 | 83% (24%) |
14.6 | 148 | 55% (2%) |
11.7 |
| “N” = Number of patients. “ORR” = Overall response rate. “CR” = Complete Response “PFS” = Progression free survival. Statistically significant results are highlighted |
||||||
| Adverse Effect | Campath | Chlorambucil |
| Infections (including CMV) | 76% | 50% |
| Lymphopenia (Grade 3 or 4) | 97% | 3% |
| Neutropenia (Grade 3 or 4) | 45% | 26% |
| Serious Adverse Effects | 44% | 20% |
| Number of patient deaths | 24 | 24 |
ASH 2024 Oral Session
Alemtuzumab (CAMPATH , MABCAMPATH ) Has Superior Progression Free Survival (PFS) vs Chlorambucil as Front-Line Therapy for Patients with Progressive B-Cell Chronic Lymphocytic Leukemia (BCLL).
Peter Hillmen, Aleksander Skotnicki, Tadeusz Robak, Branimir Jaksic, Cynthia Sirard, Jiri Mayer
Haematology, Leeds General Infirmary, Leeds, United Kingdom; Jagiellionian University Collegium Medicum, Krakow, Poland; Kopernik Memorial Hospital, Lodz, Poland; Clinical Hospital Merkur, Zagreb, Croatia; Genzyme Corporation, Cambridge, MA, USA; University Hospital Brno, Brno, Czech Republic
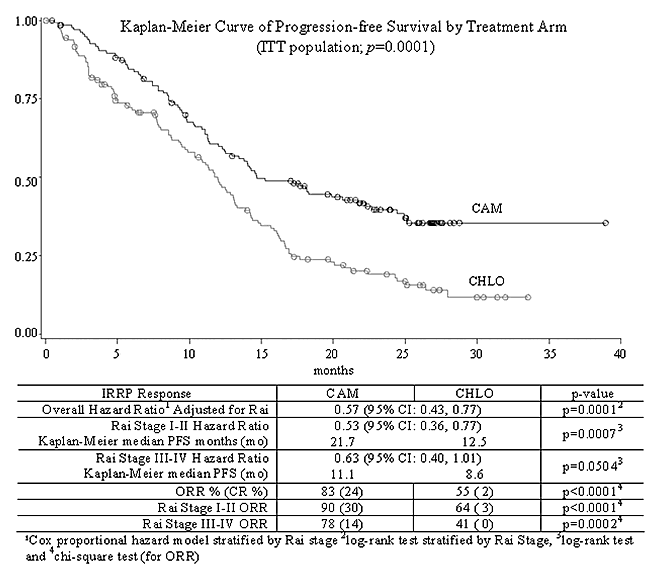
CAM307 is a phase III, open-label, multinational, randomized controlled trial comparing alemtuzumab (CAM) with chlorambucil (CHLO) for previously untreated BCLL requiring therapy. Eligible patients with Rai Stages I-IV were randomized 1:1 to either CAM 30 mg IV tiw for up to 12 weeks or CHLO 40 mg/m2 po q 28 days up to 12 cycles. CAM patients received prophylactic trimethoprim/sulfamethoxazole DS and famciclovir treatment during therapy and until CD4+ counts were 200 cells/ L. The primary endpoint was PFS; secondary endpoints were response rate, overall survival, and safety. A total of 297 patients were enrolled (CAM n=149 and CHLO n=148); median age: 60 years; performance status 0-1: 96%; maximum lymph node size 5 cm: 22%; and Rai Stage I-II: 63%, Rai Stage III-IV: 33%. Diagnosis, Rai Stage, response and disease progression were confirmed by an independent response review panel (IRRP). CAM has a significantly prolonged PFS compared to CHLO (p=0.0001; see KM survival curve).PFS in CAM vs CHLO patients with adverse cytogenetic findings of del 17p (n=21) was 10.7 mo vs 2.2 mo and for trisomy 12 (n=39) was 18.3 mo vs 12.9 mo. Results were similar for patients with del 11q (n=54, 8.5 mo vs 8.6 mo). Common ( 15%) CAM reported adverse events (AEs) (n=147), likely infusion-related, included pyrexia (70%), chills (53%), nausea (18%), hypotension (16%) and urticaria (16%). Common AEs ( 15%) in the CHLO arm (n=147) were nausea (37%) and vomiting (18%). Frequency of asymptomatic CMV viremia on the CAM arm was 52%; CMV infection occurred in only 16%, none grade 4. CAM treatment was interrupted in 56% of CMV viremic patients, and resumed in 92% with ORR 92% (31% CR). Ganciclovir was administered to 41% of CAM patients with CMV viremia. Infections, including CMV, were reported in 76% of CAM and 50% of CHLO patients while on study. Relevant grade 3/4 treatment-emergent events included (CAM vs CHLO): lymphopenia (97% vs 3%), pyrexia (8% vs 0%), CMV events (8% vs 0%) and chills (3% vs 0%). Treatment emergent grade 3/4 thrombocytopenia (16% vs 13%) and anemia (14% vs 19%) were similar, and although grade 3/4 neutropenia was more common with CAM, (45% vs. 26%), AEs of CAM vs CHLO bacteremia/sepsis (3% vs 2%) and febrile neutropenia (5% vs 3%) were comparable. Serious AEs were reported for 44% of CAM (20% for IV Ganciclovir only in CMV viremic patients) and 20% of CHLO patients while on study treatment.
CONCLUSIONS: CAM307 demonstrates that therapy-naïve BCLL patients treated with CAM have significantly longer PFS and higher ORR than those treated with CHLO, with manageable toxicities. Although CMV reactivation was reported in the CAM arm, prompt intervention maintained efficacy. The activity seen here supports ongoing investigations of CAM in first line combination therapy, high risk patients and consolidation.
Progression Free Survival by Treatment Arm
ASH 2024 Abstract
Abstract #301 appears in Blood, Volume 108, issue 11, November 16, 2024
Keywords: Phase III|Staging|Survival
Abstracts of Clinical Interest: ASH 2024 - Session Type: Poster Session, Board #270-II
Disclosures: SIRARD Medical Director at Genzyme Corporation.; HILLMEN Schering AG/Genzyme Corporation.; Sirard stock options and employee stock purchase plan at Genzyme Corporation.; Robak/Skotnicki with Genzyme Corporation; Hillmen with Schering AG.; Jaksic from Genzyme Corporation.; Hillmen Speakers Bureau for Schering AG/Genzyme Corporation.
____________
2092] Incidence of Genomic Aberrations and Associated Efficacy from a Phase III Study Comparing Alemtuzumab (CAMPATH , MABCAMPATH ) vs Chlorambucil as First Line Therapy for B-Cell Chronic Lymphocytic Leukemia (BCLL).
Tadeusz Robak, Anna Dmoszynska, Raouf Fetni, Ying Wang, Malika Belkacz, Cynthia Sirard, Mark Goldberg, Aleksander Skotnicki, Jiri Mayer, Peter Hillmen
Haematology, Kopernik Mem Hosp, Lodz, Poland; Lublin, Poland; Montreal, Canada; Cambridge, MA, USA; Krakow, Poland; Brno, Czech Republic; Leeds, United Kingdom
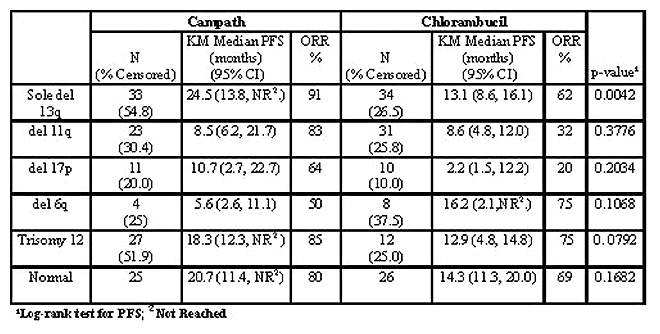
CAM307 is a randomized Phase III trial comparing the efficacy and safety of alemtuzumab (CAM) with chlorambucil (CHLO). The trial enrolled 297 previously untreated patients (pts) requiring therapy according to NCI-WG criteria. Pts were randomized 1:1 to CAM (n=149) vs CHLO (n=148) using standard dosing regimens. Fluorescence in situ hybridization (FISH) on interphase nuclei of lymphocytes isolated from blood was analyzed for cytogenetic abnormalities prior to the start of therapy. FISH analysis was performed using 13 DNA probes to detect chromosomal aberrations in 17p13.1 (P53), 13q14 (RB1, D13S319 and D13S25), 11q22.3-11q23 (ATM and MLL), 6q27 (subtelomere), 6q21 (chromosome 6q21/alphasatellite 6 cocktail probe), trisomy 8q24 (c-myc), trisomy 12 (CEP12) and translocations involving the locus of immunoglobulin heavy chain gene (IGH, 14q32.33). Samples were analyzed in 282 pts (95%); chromosomal aberrations were detected in 231 pts (82%) while 51 pts (18%) exhibited a normal interphase FISH pattern. The most frequent abnormalities were deletions (del) at loci 13q (49%), sole del 13q (24%), 11q (19%), 17p (7 %), 6q (4 %), and trisomies 12 (14%) and 8q (5%). Translocations IGH, 14q32.33 were detected in 10 pts (4%). An exploratory analysis was performed to correlate time to event variables (assessed by an independent response review panel) with cytogenetics.
Overall 165 pts (59%) revealed combination abnormalities. The most frequently observed chromosomal associations were: del 13q + del 14q (N=20, 12%), del 11q + del 13q (N=17, 10%), del 11q + del 13q + del 14q (N=11, 7%), del 11q + del 14q (N=7, 4%), trisomy 12 + del 13q (N=5, 3%), del 13q + del 17p (N=4, 2%), del 11q + trisomy 12 (N=3, 2%) and del 17p + del 6q (N=3, 2%). Coexistence of del 17p and del 11q was not observed. Although del 13q was observed with all chromosome abnormalities, nearly half of the cases del 13q14.3 (D13S25 and D13S319) coincided with an ATM deletion (11q22.3). FISH analysis has allowed the detection of uncommon abnormalities: tetraploidy (n=1), hyperdiploidy (n=1), trisomy 18 (n=1) and c-myc oncogene amplification (>15 copies per nuclei) (n=2). The latter is a well known abnormality in solid tumors but rarely seen in leukemia. In addition, del of the IGH variable region was detected in 70 pts. The biological and clinical significance of this abnormality is to be investigated.
Conclusions: Overall, 82% of treatment-naïve BCLL pts revealed cytogenetic aberrations and 59% were combination abnormalities. CAM307 demonstrates a significant improvement in PFS in pts treated with CAM vs CHLO who present with del 13q as the sole abnormality; no difference in pts with del 11q. However, a trend towards improved PFS was observed in pts with trisomy 12 and del 17p, which did not reach significance due to small sample size. Further investigation of CAM therapy in high risk cytogenetic subgroups is warranted.
Campath vs. Chlorambucil: Response by Cytogenetics
ASH Poster Session
Abstract #2092 appears in Blood, Volume 108, issue 11, November 16, 2024
Keywords: Cytogenetic abnormalities|FISH|Chromosomal translocation
Disclosures: SIRARD Medical Director at Genzyme Corporation; GOLDBERG Sr. VP, Clinical Research at Genzyme Corporation.; HILLMEN Schering AG/Genzyme Corporation.; Sirard/Goldberg stock options and employee stock purchase plan at Genzyme Corporation.; Robak/Skotnicki with Genzyme Corporation; Hillmen with Schering AG.; Jaksic from Genzyme Corporation.; Hillmen Speakers Bureau for Schering AG/Genzyme Corporation.
_________
I am always pleased when a new drug is approved for treating our guys, and Campath is certainly an important drug for CLL patients. But Campath as single agent frontline therapy? Just because you can now use it, with the FDA’s blessing, does that mean it is your best choice right of the bat? Just because you could use it, should you use it? Would you use it?
Let me see: this trial is a comparison is between Campath, the Cadillac of CLL drug therapies, the pride and joy of modern monoclonal antibody technology – and chlorambucil, one of the oldest drugs known to treat CLL. Campath therapy needs thrice weekly visits to the doctor for intravenous infusions, for a total of 12 weeks. Chlorambucil is taken as oral pills, only one day of every 4 weeks, for a total of 12 months. Campath has to be administered along with the mandated prophylactic medications for preventing bacterial and viral infections. No such precautions are generally necessary for chlorambucil. Campath (and the prophylactic medications!) costs close to an arm and a leg. Chlorambucil is off-patent, cheap, oral pills you take at home. Anybody gets the feeling this was a little loaded, a David and Goliath match-up? Do you think the choice of chlorambucil as the comparison had anything to do with the fact that it is a bit of a straw-man that is easily knocked down?
True, the Campath arm saw better overall responses and longer remissions, especially so for the tough-nut 17p deleted cases. I would have been stunned if this had not been the case. But to weigh against this was the significantly higher adverse effects associated with Campath. By now we all know that Campath is very good at killing T-cells, not just B-cells, and that it takes a long time for the T-cell counts to recover after Campath therapy (Campath Consolidation: A Home Run?). The abstract from the Karolinska Institute that was quoted in our article is quite sobering. Prior work has shown that even a year after Campath treatment critical T-cell subsets are still mere shadows of their former selves, with implications for viral infections, recurrences of skin cancer and the like. There is increasing evidence that T-cell dysfunction is linked to autoimmune disease. I wonder if there was a higher incidence of autoimmune diseases such as ITP in the Campath arm of this trial. As we pointed out in a recent article (Tregs Revealed) Campath therapy for MS patients has been linked to a higher risk of autoimmune thrombocytopenia and the drug now carries a “black-box warning label” to that effect.
Don’t get me wrong, I am fully convinced that Campath is a powerful and important drug in treating CLL, especially those that have high-risk FISH abnormalities, or late-stage patients that have failed more conventional therapies (Steroid-Campath Combinations: A Sledgehammer to Be Used Wisely). But fully 63% of the patients recruited for this trial were in early Rai Stage I-II. Only 25% of them had the dreaded 11q or 17p deletions. All of them were previously untreated. Between you and me, if you were previously untreated, early Rai stage I or II, and had the best possible FISH deletion of 13q only, would you bring out the big guns right away and opt for Campath therapy? Would that be your choice, if you had other options? Or would you be smarter if you saved the macho stuff for a later time, when you might need it more?
CLL is an incurable cancer. None of the folks in this trial were cured, whether they got low-tech chlorambucil or state-of-the-art Campath. There is not a whole heck of a lot you can do about the version of CLL you have: those are the cards you have been dealt. But there is a lot you can do in making sensible therapy choices, including saving some bullets for later on when you might need them. Just because the FDA says you can use Campath as a single agent frontline therapy, there is no law saying you have to go that way. In this case, “couldda” does not translate to “shouldda” or “wouldda”.
The following published interview with Dr. Stephan Faderl of M. D. Anderson is interesting reading. The highlighting is my effort to bring some of the juicy bits to your attention. Dr. Faderl comments that most treating physicians in the USA would not jump on Campath as the frontline drug for treating CLL. The recruitment patterns of this trial are revealing. The great majority (85%) of the patients recruited were from Eastern Europe, where I am told patients have fewer options. Clearly, not too many folks in the USA or Western Europe signed up for it. If this is called voting with their feet, I think the CLL patient community has spoken as well — and they agree with Dr. Faderl on this subject.
PDF document: Physicians' Education Resource from CancerPublications.com
Reproduced from the February 2024 issue of Cancer Abstracts and Summaries
Selected from Original Presentations at ASH 2024 Annual Meeting
Stefan H. Faderl, MD
Associate Professor of
Medicine
Department of Leukemia
The University of Texas
M. D. Anderson Cancer Center,
Houston, TX
Q: Given the results of the CAM307 study, what is the optimal way to integrate alemtuzumab into the first-line setting? Given the progression-free survival (PFS) associated with alemtuzumab in this trial, is single-agent therapy a good option for first-line therapy? Are ongoing clinical trials investigating this issue?
Dr. Faderl: CAM307 was a major randomized study
of single-agent alemtuzumab versus chlorambucil.
The trial results confirm superior outcome, both by
response rate (RR) and PFS, in the patients treated with
alemtuzumab compared to those treated with chlorambucil.
The results of this study might lead to an approval of
alemtuzumab as first-line treatment of CLL in addition to
its current indication for fludarabine-refractory patients.
Nevertheless, I think this approach is a little bit behind the
current trend of development in CLL treatment, which
clearly has been moving toward combination therapies,
most notably exemplified by the combination of monoclonal
antibodies (MoAbs) and chemotherapy (chemoimmunotherapy). Though alemtuzumab proved to be more effective than chlorambucil, that does not necessarily indicate
that alemtuzumab as single-agent therapy would be a
good option for first-line therapy. The results of this study
are interesting, but one has to recognize that chlorambucil
is not exactly very active frontline therapy, especially compared
to nucleoside analogues. Many treating physicians,
especially those in the United States, would probably
not consider chlorambucil in frontline therapy but rather
would consider agents such as fludarabine. The PFS for
the alemtuzumab-treated patients in this study was not
especially long for frontline treatment, especially compared
to experience with chemoimmunotherapy. Despite the
results of this trial, I would say that single-agent alemtuzumab
is probably not the most effective first-line option
for most patients.
As for other ongoing studies, antibody combinations such as
alemtuzumab/rituximab are being investigated as frontline
therapy in patients in the United States. One of these studies
is enrolling otherwise asymptomatic patients with high-risk
features. There are other combination studies of alemtuzumab/FCR (CFAR), which has been investigated intensively
for salvage patients and is now undergoing a clinical
trial as frontline therapy for symptomatic patients. So there
is much interest in alemtuzumab in frontline therapy regimens
but predominantly as part of a combination.
Q: Are there any patient or disease characteristics that you would use to determine if a patient is appropriate for frontline alemtuzumab
Dr. Faderl: There is clearly a trend to try to identify,
based on cytogenetic and molecular markers, groups
of patients who might benefit from specific, one could
say risk-adapted, treatment approaches. The best data to
date come from patients with 17p deletions or p53 mutations.
Although these abnormalities are rarely detected in
untreated patients, their significance increases as patients
relapse or develop resistant disease. These patients are
known to do poorly in terms of short PFS and overall
survival (OS) and poor response to fludarabine, alkylators, or rituximab.
However, good responses to alemtuzumab have been achieved in a number
of clinical studies. Part of the reason might be that alemtuzumab acts
through pathways independent of p53. This is, therefore, the first example
of cytogenetic/molecular abnormalities making a direct impact on treatment
choices. This is also a situation in which alemtuzumab might become more
important up front.
_____
Prior to publishing this article, we sent a draft version of it to members of our unofficial advisory board, experts whose names have become household words for CLL families. This is our standard operating procedure, whenever we publish an important article that may be controversial. Journals have their peer review process, we are grateful to our expert advisors for sharing their perspective, helping CLL Topics stay on message and maintain credibility. Here is the feedback we got:
______
 Enter Keywords: |
———
Disclaimer: The content of this website is intended for information only and is NOT meant to be medical advice. Please be sure to consult and follow the advice of your doctors on all medical matters.
Copyright Notice:
Copyright © 2024-2007 CLL Topics, Inc. All Rights Reserved.
All materials contained on this site are protected by United States copyright law and may not be reproduced, distributed, transmitted, displayed, published or broadcast without the prior written permission of CLL Topics, Inc. You may not alter or remove any trademark, copyright or other notice from copies of the content.
However, you may download and print material from CLLTopics.org exclusively for your personal, noncommercial use.
———