|
||||||||||
Date: March 6, 2026
by Chaya Venkat
Related Articles:
Campath: Jumping the Gun;
Steroid-Campath Combinations: A Sledgehammer;
Campath Consolidation — A Home Run?
Campath plus GCSF Can be Dangerous;
Campath Looking Better and Better
PCR Negative Remission — Is this a Cure?

There is an interesting correlation between the number of CLL research papers published each year over the last few decades and the percentage of CRs obtained by therapies available during that time period. Not all that hard to understand, it is no fun doing research on a cancer where there was little progress and patients kept dying no matter what. Now there is a smell of success in the air, a real chance that we may be able to prolong life, perhaps even cure some of the patients. CLL is becoming a fashionable cancer to study. The focus on CLL and other cancers of the immune system (the AIDS epidemic had something to do with it as well) is paying off handsomely, extrapolating as well into other immune system related areas such as Lupus, MS, diabetes, rheumatoid arthritis, even the "heartbreak of psoriasis".
Sometimes it helps to look back to see how things were done in the past. We can see just how far we have come (and how far we have yet to go!) Not that long ago, in your father's generation, the standard of care for newly diagnosed CLL patients was to do nothing until the symptoms got really bad. The official label for this is "Watch & Wait", and it was in keeping with the physician's oath "Above all, do no harm". Jump starting therapy made no sense when there were few options - very often the drugs available back then created more problems than they solved.
Along came purine analogs in the early 1980s, the most famous of them being fludarabine ("Fludara"). Monoclonal antibodies came on the scene in the late 1990s. Suddenly, therapies based on combinations of fludarabine with monoclonal antibodies like Rituxan and Campath are all the rage now since they increase the response rates dramatically. CR ("complete response") rates went from 10% with chlorambucil to 60%-70% with the newer drug combos using fludarabine and monoclonal antibodies. Even more encouraging, some of these CRs were really robust responses. A significant percentage of lucky patients had no trace of detectable disease even when tested with our best and most sensitive methods.
Not only are we seeing a real change in the nature of the drugs and therapies, we are also getting more ambitious in how we define success. As things stand, the official designation of "CR" can be given even when the patient has as much as 30% lymphocytes in the bone marrow. Hardly a "complete response", from a layperson perspective! Similarly, patients with little nodules in their bone marrow biopsy are considered to be in partial nodular remission ("NPR"), with no distinction being made between real CLL cells in the nodules versus non-cancerous T-cells. In other words, many patients considered to be in CR by current criteria can still harbor a lot of leukemic cells in their bone marrow, whereas others who are deemed to be in partial response ("PR") may be a lot closer to a "real" CR.
Another fly in the ointment in classifying responses is the severity of side effects caused by some of these aggressive combo therapies. They have more punch, but at the cost of increased hematological toxicity. Cytopenias such as neutropenia and anemia, for example, are pretty common. Patients with these therapy-related problems are classified as PRs, even if there is no trace of CLL left behind. It might make sense to mandate an official waiting period so that patients have a chance to recover from treatment-related cytopenias before evaluating their response and so that these often short-lived cytopenias do not get in the way of accurate classification of their CLL response.
Reflecting our new found optimism, well regarded researchers are suggesting that we expand the response classification to include MRD-negative CR. This means no trace of disease, no "Minimum Residual Disease", even when we look for it with our best methods. Obviously, we need to standardize the methods used to look for MRD. The two contenders right now are allele-specific polymerase chain reaction ("PCR") and four-color cytometry. The time has come to include MRD-negative CR (also called PCR negative status) in the line-up of response classifications. Next stop, I would like to see the word "CURED" in the official list of possible responses to therapy. You agree? I thought you would.
This is a tough question to answer. The good news is that we are beginning to chip away at the problem and coming up with useful answers. Below is the abstract of an excellent paper that has just been published in the Journal of Clinical Oncology. I will review the highlights of it for you, but if you are contemplating Campath therapy (do remember, Campath and "Alemtuzumab" are the same thing) in the near future you may want to read the full article. Write to us and we will help you locate the full text version of the article. This edition of the JCO also has a terrific editorial by Emili Montserrat, with overview comments on the Moreton paper. It is worth reading as well.
Journal of Clinical Oncology, Volume 23 Number 13 - May 1, 2026
Eradication of Minimal Residual Disease in B-Cell Chronic Lymphocytic Leukemia After Alemtuzumab Therapy Is Associated With Prolonged Survival
Paul Moreton, Ben Kennedy, Guy Lucas, Michael Leach, Saad M.B. Rassam, Andrew Haynes, Jane Tighe, David Oscier, Christopher Fegan, Andy Rawstron, and Peter Hillmen
PURPOSE: To test whether eradication of minimal residual disease (MRD) in B-cell chronic lymphocytic leukemia (CLL) by alemtuzumab is associated with a prolongation of treatment-free and overall survival.
PATIENTS AND METHODS: Ninety-one previously treated patients with CLL (74 men and 17 women; median age, 58 years [range, 32 to 75 years]; 44 were refractory to purine analogs) received a median of 9 weeks of alemtuzumab treatment between 1996 and 2026. Regular bone marrow assessments by MRD flow cytometry were performed with the aim of eradicating detectable MRD ( 1 CLL cell in 105 normal cells).
RESULTS: Responses according to National Cancer Institute-sponsored working group response criteria were complete remission (CR) in 32 patients (36%), partial remission (PR) in 17 patients (19%), and no response (NR) in 42 patients (46%). Twenty-two (50%) of 44 purine analog-refractory patients responded to alemtuzumab. Detectable CLL was eradicated from the blood and marrow in 18 patients (20%). Median survival was significantly longer in MRD-negative patients compared with those achieving an MRD-positive CR, PR, or NR. Patients achieving an MRD-negative CR had a longer treatment-free survival than patients with MRD-positive CRs, PR, or NR: MRD-negative CRs, not reached; MRD-positive CRs, 20 months; PRs, 13 months; NR, 6 months (P .0001). Overall survival for the 18 patients with MRD-negative remissions was 84% at 60 months. Eight (47%) of the MRD-negative patients converted to MRD positivity at a median of 28 months.
CONCLUSION: MRD-negative remission in CLL is achievable with alemtuzumab, leading to an improved overall and treatment-free survival.
In a nutshell, the Moreton paper tries to answer two important questions:
In this cohort of pretty heavily pre-treated patients with poor prognosis, the answer to both questions seems to be a very encouraging "Yes". Now for the details - what makes this study valuable and the results credible.
This study covers a time span of 7 years, from 1996 to 2026. Ninety one previously treated CLL patients received Campath therapy (an average of 9 weeks of therapy). You know what they say, the laughs you get as a stand-up comic depends on how tough the audience is. The same concept applies in CLL clinical trials as well. We know all too well that there are many flavors of CLL, some are easy to deal with and some are tough S.O.B.s right from the start. The patient cohort of this clinical trial was a tough crowd. 44 out of the 91 of the patients were tagged with the ominous label "fludarabine refractory". Some of the other patient characteristics are given in the table below. This is how the patients stacked up, before the start of this study. As you can see, roughly half the patients were in Rai stage IV, two-thirds had swollen lymph nodes, a similar number had a swollen spleen and just about half had flunked fludarabine. The majority of them had been through several layers of therapy prior to getting into this clinical trial. There are a lot more details given in the full text article.
Characteristics |
Number |
% of |
|
All Patients |
|
91 |
100 |
Age
|
More than 60 |
39 |
43 |
Less than 60 |
52 |
57 |
|
Rai Stage
|
0/I |
13 |
14 |
II |
23 |
25 |
|
III |
10 |
11 |
|
IV |
45 |
49 |
|
Size of
|
None |
33 |
36 |
Less than 5 cm |
47 |
52 |
|
More than 5 cm |
11 |
12 |
|
Swollen
|
Yes |
57 |
63 |
No |
34 |
37 |
|
Swollen
|
Yes |
10 |
11 |
No |
81 |
89 |
|
B-symptoms?
|
Yes |
15 |
17 |
No |
76 |
83 |
|
Fludarabine
|
Non-responsive |
44 |
48 |
Responsive |
44 |
48 |
|
Never had any |
3 |
4 |
|
The idea behind this study was to take as many of these people as possible into squeaky clean MRD negative CR and to see if this prolonged overall survival as well as remission period. Regular bone marrow biopsies were done and four color flow cytometry were performed with the aim of determining level of detectable minimum residual disease. (Don't get confused by terminology, some of you may be more familiar with the term "PCR" response, it means pretty much the same thing, in this case the PCR methodology is used to verify the level of residual disease. Four color flow cytometry is another technique to look for MRD - we could just easily call this level of response as "flow cytometry negative". "MRD negative" is more general term, since it does not depend on the actual method used to look for the last traces of CLL.)
Patients got Campath 30 mg three times a week until they got the best possible response for that patient. Patients with a mere "CR" by full blown blood and bone marrow examination continued getting Campath until either the CLL or the patient cried "uncle!" In other words, therapy was continued until the patient achieved the desired goal of "MRD negative CR" response with no sign of the CLL anywhere; with the condition that therapy was stopped if the patient's disease got worse or if he/she had trouble tolerating it. 84 patients got intravenous Campath and seven patients received it as a subcutaneous injection.
Campath therapy is no cake walk. The precautionary measures taken in this study to protect patients may well form the definition of "best practices" in Campath therapy. For that reason, I will report the details here - you can use this as a cheat sheet to make sure you are getting the right protection when it is your turn to step up to Campath therapy.
The table below summarizes the infusion related complications, as well as infections during the Campath therapy. Bear in mind these patients have all been through a lot therapy already, hardly your average healthy chemo naive patient group. Even with that caveat, it is sobering to see the high percentage of patients who had infusion related problems. I wonder if these stats would have been different if all of the patients got subcutaneous Campath injections rather than the intravenous infusions. I guess the earlier patients in this study (remember, this study ran for 7 years, from 1996 through 2026) got intravenous infusions before researchers figured out the sub-q versions is easier to take.
Complication |
NCI Toxicity |
NCI Toxicity |
Fever |
63% |
13% |
Rash |
27% |
1% |
Nausea |
31% |
0% |
Hypotension |
13% |
4% |
Hypoxia |
3% |
3% |
Pulmonary infection |
3% |
7% |
Febrile neutropenia |
3% |
4% |
Herpes |
3% |
2% |
CMV |
2% |
6% |
Fungal infection |
0% |
3% |
This represents all infections during and up to 1 month after completion of Campath therapy. |
||
Less frequent adverse events included fatigue (11%), headache (4%), dizziness (3%), bronchospasm (2%), and diarrhea (2%). Most often things got better by the end of week 3 of therapy. Not included in the table above, 48% of the patients had neutropenia (below 1,000 counts), and 30% had neutrophil counts dive below the 500 level. 20% of the patients needed the Neupogen support. Thrombocytopenia (low platelet counts) occurred in 73% of the patients, and in 46% of the patients platelet counts got lower than 50. Some of the patients were taking corticosteroids (prednisone and the like), 18 out of 31 patients (58%) who used corticosteroids developed infections, compared to the much lower number of 21 out of 60 (35%) who did not get steroids. It seems clear, patients who received steroids along with Campath were more likely to get infections.
Now that we have waded through all the side effects and complications, time to see the good stuff. Was there bang for the buck, reason to justify going for squeaky clean, no trace left, MRD negative CR response? Pictures tell the story a lot better than words and I borrowed two of the graphs in the paper.
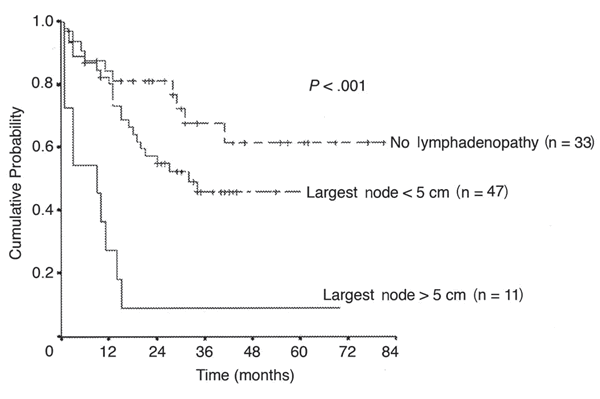
It is very clear by now that the Achilles' heel of Campath therapy is swollen lymph nodes. Guys with baseball size lymph nodes (actually, 5 cm or larger) did not do so well. If there were no swollen lymph nodes, or they were modest size, the response to Campath therapy was much better. Moral of the story, you may want to consider other options if you have king-size nodes. The overall survival for folks with large lymph nodes is hardly enough to justify the downside of Campath therapy, there were hardly any of these guys left to tell the story a year later. Not so the folks with small or no lymph nodes. I am particularly interested to see the flattening out of the survival curves, suggesting that past the 3-4 year mark these folks were home free, maybe. Dare we say, "Cured"?
Overall Survival, by Size of Largest Lymph Node
Moreton, et al., JCO
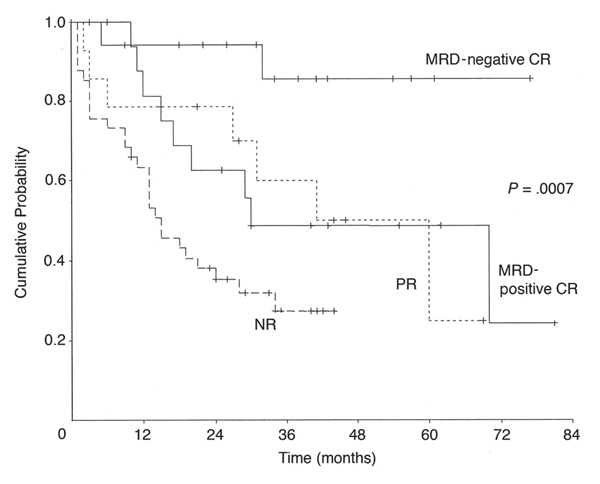
How does getting an MRD negative CR response strategy work out, as compared to pulling the plug on therapy a little sooner? There is a startling difference between patients who got an MRD negative CR, versus those that got a CR, but not good enough to be MRD negative. In other words, if you are tolerating the therapy pretty well and the response is looking good, it might be worthwhile to hang in there and go an extra few rounds until you got the desirable MRD negative CR. As you can see, close to 90% of people with MRD negative response were still around, 6 to 7 years later. Not bad at all, for a heavily pretreated and late Rai stage group of patients!
Overall Survival, by Response to Campath Therapy
Moreton, et al., JCO
Below is an abbreviated chart showing how patients with different characteristics going into this Campath clean-up did. You will have to read the full text article to get all the details. Looking at the half-full glass for a change, it is encouraging to know that a significant portion of "tough cases" - older patients, patients with late Rai-stage disease, even those who have flunked fludarabine therapy earlier on - achieved the coveted MRD negative CR response. Before the advent of monoclonals like Campath, there was a scary feeling to the words "fludarabine refractory". There were few good options past that point, with the exception of bone marrow transplants. I expect that the majority of patients who have developed drug resistance to fludarabine are "Bucket C" folks with ATM (11q) or p53 (17p) abnormalities, and few drugs worked for them. The difference now is that we have Campath, which seems to be able to kill CLL cells with or without the help of the ATM and p53 genes. Before the advent of Campath, less than 10% of fludarabine refractory patients would be expected to survive 5 years, and the most startling finding of this study is this: even in fludarabine refractory patients, those that got MRD-negative response are surviving much longer!
Characteristics |
# Patients |
NR |
PR |
CR MRD +ve |
CR MRD -ve |
|
All Patients |
|
91 |
|
|
|
|
Age
|
More than 60 |
39 |
23 |
6 |
3 |
7 |
Less than 60 |
52 |
19 |
11 |
11 |
11 |
|
Rai Stage
|
0/I |
13 |
0 |
4 |
5 |
4 |
II |
23 |
11 |
5 |
3 |
4 |
|
III |
10 |
5 |
0 |
2 |
3 |
|
IV |
45 |
26 |
8 |
4 |
7 |
|
Size of
|
None |
33 |
4 |
5 |
11 |
13 |
Less than 5 cm |
47 |
28 |
11 |
3 |
5 |
|
More than 5 cm |
11 |
10 |
1 |
0 |
0 |
|
Fludarabine |
Non-responsive |
44 |
22 |
10 |
4 |
8 |
Responsive |
44 |
18 |
7 |
10 |
9 |
|
There are several things I like about this paper. The authors are to be congratulated for staying the course. It is no easy job to run a clinical trial that stretches over 7 years. I also liked the detailed characterization of the patient group. If one were to stoop to the practice of "cherry-picking", it is all too easy to get together a patient cohort that is "easy", report eye-popping statistics without going into all the caveats. This study is not a full-blown phase III trial either, which would need a well-matched control group. Frankly, it is becoming increasingly difficult to do such comparative studies. How can one ethically deny patients in the control group access to drugs like Campath, when it is becoming clear that they can give good responses as well as increase overall survival? As a single-arm study, the fact that the patient cohort in this study was such a difficult one and well characterized is very important in judging the results. That a significant number of fludarabine refractory patients were able to get MRD negative CRs is truly impressive, and the statistical significance of this result cannot be denied.
The infusion related toxicities, infections and cytopenias are sobering, I am glad to see the details of the protective measures taken spelled out. If your local oncologist is a little lax on the details of how Campath should be administered, I suggest you make him a present of the hard copy of the full text Moreton, et al., paper.
I know several of the authors of this paper have also published on the topic of intravenous infusion of Campath compared to subcutaneous injections. Presumably the patients recruited earlier in this study did not get the benefit of hindsight. It seems clear that sub-q administration of Campath is a lot easier on patients. I wish the authors had taken the trouble to spell out this important detail, in this paper as well.
There is one definite negative result in this study. Patients with large lymph nodes do not do well on Campath therapy, as it is currently practiced. That is too bad, because a very substantial percentage of patients with poor prognosis or late stage disease have large lymph nodes. What are the options for these patients? I hope the researchers are looking at ways of improving responses in such cases. New research reports suggest it might be possible to flush out CLL cells out of the lymph nodes and into the peripheral blood, by means of using mobilizing agents such as G-CSF, GM-CSF, AMD-3100, or by interfering with homing mechanisms that use SDF-1/CXCR-4 interactions. Is the problem one of access, a case of the fat lady not being able to get in through a narrow door? Do bulky lymph nodes pose a problem because the large Campath molecules cannot get in through all the nooks and crannies of lymph node architecture? Or is the problem due to "nurse like cells" and the microenvironment of the lymph nodes that makes it harder to kill CLL cells? Do we need to make the next generation Campath-like drugs smaller molecules so that they can get in more easily, or do we have to make the drugs more potent so that even nurse-like cells cannot protect CLL cells from death?
We have reviewed in a previous article the interesting work coming from Long Island Jewish Hospital. The "heavy water" experiment reported by Chiorazzi, et. al. has shed interesting light on the life of CLL cells. It seems they are not quite as indolent as we thought. They multiply at a pretty fast clip. Knowing details like this is very important if we are to have a chance of improving therapy options. Campath is an extremely important drug in our arsenal and I am dismayed we still do not have a good handle on the pharmacokinetics of this monoclonal. Berlex / Schering AG and Genzyme/Ilex would be well served to fund such studies. Yeah, I know all the excuses, red tape, expenses, it is hard to get human volunteers for such studies, yada yada. But what is the excuse for not doing detailed studies of this sort, with radio-labeled Campath in mouse or primate studies? It is no longer good enough to say Campath does not work very well for lymph nodes, we need to know more about why this is so, and come up with ways to get around the problem.
All in all, a credible and well conducted study by some of the best experts in the field, all the more valuable because the full details have been promptly reported. Take a bow, gentlemen.
 Enter Keywords: |
———
Disclaimer: The content of this website is intended for information only and is NOT meant to be medical advice. Please be sure to consult and follow the advice of your doctors on all medical matters.
Copyright Notice:
Copyright © 2026-2007 CLL Topics, Inc. All Rights Reserved.
All materials contained on this site are protected by United States copyright law and may not be reproduced, distributed, transmitted, displayed, published or broadcast without the prior written permission of CLL Topics, Inc. You may not alter or remove any trademark, copyright or other notice from copies of the content.
However, you may download and print material from CLLTopics.org exclusively for your personal, noncommercial use.
———