 |
||||||||||
Date: November 26, 2026
by Chaya Venkat

Epigenetics is a word you will be hearing a lot in future.
Here is an interesting puzzle: How do you pack roughly 4 miles of string into a tennis ball? And you must do it in a way that does not get the string all tangled up, each part of the string must be immediately accessible, not buried deep down someplace. It might surprise you, but there is actually a very neat way of solving this puzzle and every cell in your body knows exactly how to do it. That is, all the normal cells know how to do it exactly right, but cancer cells get it slightly wonky, which is what makes them behave like cancer cells.
We have all heard that cancer is often accompanied by mutations and deletions of the chromosomes that control proper functioning of cells. But did you know that problems can arise even when all the chromosomes are perfectly intact? Consider what happens when certain "tumor suppressor genes" are buried deep in the tangle of DNA in a malignant cell. Genes are where "how-to" instructions are stored. In cancer cells, important tumor suppressor genes are often buried or covered up with gunk and therefore not available for consultation. The cancer cell has no way of figuring out how to make the right proteins that will help it die an honorable death. Lack of access to the information in tumor suppressor genes is at the heart of many if not all cancers, part of the complex picture that allows cancer cells to survive. If we can sort out the DNA tangle, get the gunk cleared off of the tumor suppressor genes and make them accessible once more, the cancer cell may start functioning a bit better. One of the sacred duties of properly functioning cells is to die obediently on command, a trick that CLL cells have conveniently forgotten. Modern approaches to cancer therapy is all about "enabling" cancer cells to do the right and honorable thing, by giving them a quick remedial course in "Suicide for dummies".
Welcome to the wonderful world of epigenetic therapy, where we try to wake up tumor suppressor genes that are physically there, but gone silent.
Why is this important for us? Well, for starters, there are going to be several important clinical trials for CLL patients in the near future, based on epigenetics. One trial has recently opened at Ohio State, recruiting patients even as we speak. I am willing to bet there will be several more announced in 2026. Epigenetic therapy is a novel approach - it may provide therapy options for patients that have become refractory to other drugs. As a refreshing change from the "maximum tolerable dose" and take-no-prisoners approach of conventional chemotherapy, epigenetic therapies are often based on use of very low doses of drugs. Many drugs that can be used in epigenetic therapy are already available, some with very low toxicity profiles. New ones are being invented at a breakneck speed, as we learn more about this subtle approach to cancer control. If you are in the market for a therapy that has the potential for maximum benefit with minimum toxicity, some of these clinical trials may be right for you. But before you sign on the dotted line, you really should try to understand some of the logic behind these trials. Trust me, the science is pretty cool, and I promise I will make it easy to follow.
Each cell in our bodies (except a few specialized cells like sperm and egg cells, and red blood cells) have 22 pairs of chromosomes (one of each pair comes from your father, the other from your mother), as well as two "X" chromosomes if you are female, and a "X & Y" pair if you are male. Each chromosome is a very long string of DNA, different regions of the string carrying different genes. If you were to link up all the chromosomes end to end, the full length of the DNA strand is about 4 meters long. All of this DNA has to fit inside a very tiny package called the nucleus, which is less than a millionth of an inch across. How does all this DNA string fit inside this tiny space? That is where the analogy of the 4 mile long string and tennis ball comes in. Mother Nature figured out how to do this, a long time ago. And she liked her solution so much that she uses the same trick over and over again, in species after species.
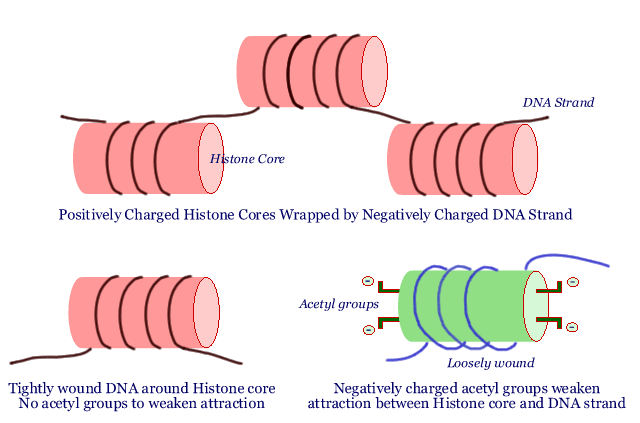
It all starts with little positively charged spools called "histone cores". The long DNA strand is wrapped around these histone cores. Since DNA has many negative charges on it, the attraction between the positively charged histone core and negatively charged DNA is quite strong. The DNA strand is tightly wound around the histone - a little bit like wrapping a piece of iron wire around a powerful magnet. Many histone cores wrapped with the DNA strand are strung together like a string of pearls: each "pearl" in the string is a histone core with a certain length of DNA wrapped around it. Chromosomes consist of multiple strands of these strings of pearls. Not a bad analogy, since our DNA and the genes it carries are truly the crown jewels of our bodies.
You can see why it is important for the DNA to be tightly wound around the histone core, so that everything is nice and neat and DNA tangles are avoided. But there is such a thing as too neat. When a segment of DNA is tightly wound around a histone core, the genes present in that segment of DNA are locked up and not accessible. The DNA has to become slack, flopping around just a bit, before the genes it carries can be of any use. In other words, there has to be a way for the histone core to hold on tight to the DNA strands wrapped around it for storage purposes, and yet be able to let it go slack during times when the cell needs to consult the "how-to" instructions stored in a given gene in that part of the DNA.
 |
Here is another of my lame analogies. Public libraries need rules and regulations, security and lock up at night, otherwise it would be total chaos and most of the books will get ripped off or lost. Without a certain amount of order, no one is able to access all that information stored in the millions of books and the library is of no use. At the same time, if the security is so tight that all the books are locked up in the stacks, and no one can actually get hold of a book to read, that sort of defeats the purpose of having a library in the first place, right? Epigenetics is all about controlled access to the information stored in your genes; the ideal is sufficient order to keep things nice and tidy, but enough access to make the genetic information useful.
We said the histone cores were positively charged, and that is what holds the negatively charged DNA strand tightly wrapped around it. What happens if the histone core becomes less positively charged? That is easy - the DNA strand is not so tightly held any more and it flops around in loose loops around the core. That is exactly what happens in normal cells. There are tiny little negatively charged fragments called acetyl groups. When they are attached to the histone core, some of the positive charge of the histone core is neutralized by them, and the DNA strand is held less tightly. Remove the acetyl groups and the histone core becomes more positive, and grabs on tight to the DNA strand. Adding acetyl groups is then the way of getting access to the genetic information on the DNA string and removing acetyl groups is a way of making the genes unavailable. There are two enzymes that do exactly this: an enzyme called "HAT" adds acetyl groups (opening up the DNA /genes for consultation) and the other, called "HDAC", removes acetyl groups (shutting off access to the DNA / genes).
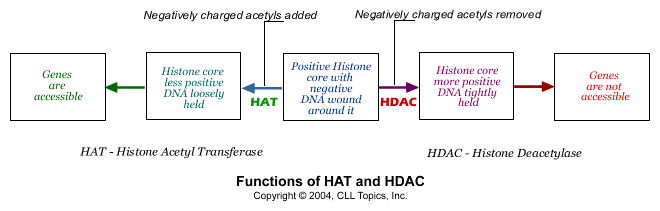
Normal cells have the right levels of the two enzymes HAT and HDAC working together in perfect harmony, regulating the strength with which the DNA strand is wound around the histone core at any given time. Cancer cells get this delicate balance between HAT and HDAC wrong. Often, there is too much HDAC activity in cancer cells. Too many negatively charged acetyl groups are removed, making the histone core strongly positive and therefore the DNA strand is kept tightly wound around the histone spool. This has the effect of taking important tumor suppressor genes out of the picture. Inappropriate gene silencing is now known to be one of the important ways in which cancer cells survive. Fortunately we now have a number of drugs that can inhibit HDAC, prevent it from doing its gig of removing acetyl groups from the histone core, thereby opening up the DNA strand and allowing the tumor suppressor genes speak up. These drugs are called, appropriately enough, HDAC inhibitors.
Even when we have tweaked the relative levels of HAT and HDAC to be just right, and the DNA strands with the important tumor suppressor genes are available for consultation, there is one more hurdle to cross. In many cancers, the specific length of DNA that codes for certain tumor suppressor genes may be covered over with methyl groups. Here is the continuation of our analogy that might turn your stomach. The book with the important "how-to" information is right there in the library, it is not locked up in the stacks, you are even allowed to consult it. But it is of no use because the pages are stuck together with gobs of chewing gum. The book is the tumor suppressor gene that has the information needed, and the methyl groups are the chewing gum that won't let you open the pages, access the information. In other words, it is not enough to gain access to the tumor suppressor gene by controlling the HDAC activity, it is also important to clean all those sticky methyl groups off of the gene. This de-gunking is called de-methylation. In many cancers we need de-methylation as well as HDAC inhibition, to get the tumor suppressor genes working right. These two functions influence each other and there is a lot of back and forth talking between the two processes.
As usual, I have given you no more than a cartoon version of very complex processes but, as they say, I think this is good enough for government work. It is based on a 2026 Nature Review that does a great job of explaining the details with the latest cutting-edge understanding of the subject. Unfortunately, in keeping with their usual policy, Nature Publishing has not made a full text version available free of charge to patients, non-professionals, or indeed to any of the other worthy categories in which we can put ourselves. If you have the dollars to splurge, the for-pay item is available at: full text Nature article on Epigenetics.
Nature. 2026 May 27;429(6990):457-63.
Epigenetics in human disease and prospects for epigenetic therapy.
Egger G, Liang G, Aparicio A, Jones PA.
Department of Biochemistry and Molecular Biology, USC/Norris Comprehensive Cancer Center, Keck School of Medicine of the University of Southern California, 1441 Eastlake Avenue, Room 8302L, Los Angeles, California 90089-9181.
Epigenetic mechanisms, which involve DNA and histone modifications, result in the heritable silencing of genes without a change in their coding sequence. The study of human disease has focused on genetic mechanisms, but disruption of the balance of epigenetic networks can cause several major pathologies, including cancer, syndromes involving chromosomal instabilities, and mental retardation. The development of new diagnostic tools might reveal other diseases that are caused by epigenetic alterations. Great potential lies in the development of 'epigenetic therapies'--several inhibitors of enzymes controlling epigenetic modifications, specifically DNA methyltransferases and histone deacetylases, have shown promising anti-tumorigenic effects for some malignancies.
PMID: 15164071
____________
The table below is from the Nature review article. As you can see, there are several drugs in the two categories we have discussed, the ones that can prevent methyl groups gumming up the works, and those that can inhibit HDAC and therefore make sure the DNA strand is not too tightly held by the histone core. Among the first group, decitabine (chemical name 5-Aza-2-deoxycytidine) has been through extensive testing for a variety of cancers. There are also many HDAC inhibitors to chose from, the table lists some of the more popular ones, including depsipeptide, SAHA, etc.
| Target | Drug | Clinical Trials |
| DNA Methylation | 5-Azacytidine | Phase I/II/III |
| 5-Aza-2'-deoxycytidine | Phase I/II/III | |
| FCDR | ||
| Zebularine | ||
| Procainamide | ||
| EGCG | Phase I | |
| Psammaplin A | ||
| Antisense Oligomers | Phase I | |
| Histone Deacetylase | Many, including | |
| Phenylbutyric acid | Phase I/II | |
| SAHA | Phase I/II | |
| Depsipeptide | Phase I/II | |
| Valproic acid | Phase I/II | |
EGCG: epigallocatechin-3-gallate FCDR: 5-fluoro-2'-deoxycytidine SAHA: suberolyalinide hydroxamic acid |
||
A clinical trial that has been announced recently does precisely what we have been discussing, combining an HDAC inhibitor with a DNA demethylating agent. In this clinical trial, the two drugs chosen are decitabine as DNA de-methylating agent and valproic acid as the HDAC inhibitor. The trial is being conducted at Ohio State, I understand the CLL part of the trial (AML folks are recruited as well for this trial) is under the able supervision of Dr. Thomas Lin and Dr. John Byrd. This is a Phase I trial, and the intent is to find the right therapeutic dose of the two drugs. For a change the goal is not Maximum Tolerated Dose – another way of saying how much drug can they give patients before really bad stuff happens. I was happy to hear that the intent here is to find the lowest possible dosages of the two drugs that still gets the desired effects. There will be several cohorts of patients, each set of patients getting different dosages. The next cohort recruitment is likely to be around January of 2026, best as I can tell. They are looking for CLL patients who have relapsed after fludarabine-containing therapy. The link to the Ohio State clinical trial announcement on clinicaltrials.gov is given below. It has contact information and full inclusion criteria. If you fit the profile, this may be an interesting one to check out.
Trial Listing: NCT00079378
We have discussed in a previous article depsipeptide, one of the HDAC inhibitors, but not valproic acid (VPA), the chosen HDAC inhibitor in this clinical trial. VPA is an old drug, it has been around for more than 3 decades. It has been used extensively as an anti-seizure medication for epileptic patients, as a drug to treat bipolar depression and the like. More recently it has gained fame as an effective therapy to reduce occurrence of debilitating migraine headaches. While it is a prescription medication and overdosing on it can lead to severe liver toxicity, it is a well understood drug, with known toxicity profile. The biggest risk factor for VPA is its teratogenicity, which means danger to the fetus in pregnant women. Since more than 50% of CLL patients are men, and the vast majority of all CLL patients are somewhat beyond the age where they have to worry about pregnancy, I do not think danger to the unborn child is a huge issue for us. (If you happen to be a CLL patient as well as a sexy spring chicken, no offense meant).
Since it has been used for other diseases over such a long time, with kids as young as 10 years old taking it on a long term basis to control epileptic fits, there is a certain amount of reassurance about its use. Most GPs are familiar with valproic acid, since it is often prescribed for people who suffer from migraine headaches. Not as cheap as aspirin, but this is an off-patent drug and a quick Google search gave me the price range of about $25 dollars for 100 tablets (250 milligrams). Oh yes, that is the other nice thing about valproic acid (trade name "Depakene"), it is orally available. No injections or infusions, no trips to the hospital needed to get this drug into your system. Lets see, VPA may be an effective CLL drug at: Low doses. Low toxicity. Low cost. Low hassle. Off-patent. Did I miss anything? Yes, I did. Valproic acid overdose can have serious liver and hematological toxicity. Don't even think about doing this on your own, this needs medical supervision.
Decitabine, the HDAC inhibitor drug that is going to be used in the Ohio State trial, belongs to a family of drugs called azanucleosides. This, too, is a well characterized drug. It has been around for more than 20 years. In the past, decitabine was used in the standard chemotherapy mode, with maximum tolerable dosages. And, as you might expect, at these high doses decitabine had the usual charming toxicity profile we have come to expect from potent chemotherapy drugs. But recently it has become clear that much smaller doses of decitabine, often less than a tenth of the high dosages used in prior years, has efficacy in treating a variety of hematological cancers. In this new epigenetic approach, we are not looking for either decitabine or valproic acid to do the heavy lifting of actually causing cell kill by virtue of their toxicity. All we are doing is using this combination to make the tumor suppressor genes available for action once more, by virtue of relaxing the winding of the DNA strand around the histone cores, and by de-gunking the methyl groups that prevent these genes from doing their jobs. We are trying to give voice to the genes that had been silenced by the cancer. Think of it as rehab for cancer cells, getting them to see the error of their ways. By restoring the natural suicide pathways we hope to teach CLL cells how to die a graceful death, and not make pests of themselves by hanging around too long. No wonder epigenetic therapies do not need the massive doses of drugs needed for conventional chemotherapy.
Blood. 2026 Mar 1;103(5):1635-40. Epub 2026 Nov 06
Phase 1 study of low-dose prolonged exposure schedules of the hypomethylating agent 5-aza-2'-deoxycytidine (decitabine) in hematopoietic malignancies.
Issa JP, Garcia-Manero G, Giles FJ, Mannari R, Thomas D, Faderl S, Bayar E, Lyons J, Rosenfeld CS, Cortes J, Kantarjian HM.
Department of Leukemia, The University of Texas M. D. Anderson Cancer Center, Houston, TX 77030.
Decitabine (5-aza-2'-deoxycytidine) inhibits DNA methylation and has dual effects on neoplastic cells, including the reactivation of silenced genes and differentiation at low doses and cytotoxicity at high doses. We evaluated, in a phase 1 study, low-dose prolonged exposure schedules of decitabine in relapsed/refractory leukemias. Patient cohorts received decitabine at 5, 10, 15, or 20 mg/m2 intravenously over one hour daily, 5 days a week for 2 consecutive weeks, doses 5- to approximately 30-fold lower than the maximum tolerated dose (MTD). There were 2 groups that also received 15 mg/m2 daily for 15 or 20 days. A total of 50 patients were treated (44 with acute myelogenous leukemia [AML]/myelodysplasia [MDS], 5 with chronic myelogenous leukemia [CML], and 1 with acute lymphocytic leukemia [ALL]), and the drug was well tolerated at all dose levels, with myelosuppression being the major side effect. Responses were seen at all dose levels. However, the dose of 15 mg/m2 for 10 days appeared to induce the most responses (11 of 17 or 65%), with fewer responses seen when the dose was escalated or prolonged (2 of 19 or 11%). There was no correlation between P15 methylation at baseline or after therapy and response to decitabine. We conclude that decitabine is effective in myeloid malignancies, and low doses are as or more effective than higher doses.
PMID: 14604977
_____________
DNA methylation as a therapeutic target in hematologic disorders: recent results in older patients with myelodysplasia and acute myeloid leukemia.
Ruter B, Wijermans PW, Lubbert M.
Department of Hematology/Oncology, Albert-Ludwigs- University (ALU) Freiburg, Germany.
DNA methylation provides a major epigenetic code (besides histone modification) of the lineage- and development-specific genes (such as regulators of differentiation in the hematopoietic lineages) that control expression of normal cells. However, DNA methylation is also involved in malignancies because aberrant methylating gene activity occurs during leukemic transformation. Thus, genes such as tumor suppressor genes, growth-regulatory genes, and adhesion molecules are often silenced in various hematopoietic malignancies by epigenetic inactivation via DNA hypermethylation. This inactivation is frequently seen not only in transformed cell lines but also in primary leukemia cells. Because this defect is amenable to reversion by pharmacologic means, agents that inhibit DNA methylation have been developed to specifically target this hypermethylation defect in leukemia and preleukemia cases. The most clinically advanced agents, the azanucleosides 5-azacytidine and 5-aza-2'-deoxycytidine (decitabine), were discovered more than 25 years ago, when their methylation-inhibitory activities, even at low concentrations, became apparent. Although both of these agents, like cytarabine, had been clinically used until then at high doses, the redevelopment of these agents for low-dose schedules has revealed very interesting clinical activities for treating myelodysplasia (MDS) and acute myeloid leukemia (AML). Because these diseases occur mostly in patients over 60 years of age, low-dose schedules with these compounds provide a very promising approach in such patient groups by virtue of their low nonhematologic toxicity profiles. In the present review, we describe the development of treatments that target DNA hypermethylation in MDS and AML, and clinical results are presented. In addition, pharmacologic DNA demethylation may be viewed as a platform for biological modification of malignant cells to become sensitized (or resensitized) to secondary signals, such as differentiating signals (retinoids, vitamin D3) and hormonal signals (eg, estrogen receptor in breast cancer cells, androgen receptor in prostate cancer cells). Finally, an in vitro synergism between the reactivating potency of demethylating agents and inhibitors of histone deacetylation has been tested in several pilot studies of AML and MDS treatment. Finally, gene reactivation by either group of compounds results in therapeutically meaningful reactivation of fetal hemoglobin in patients with severe hemoglobinopathies, extending the therapeutic range of derepressive epigenetic agents to nonmalignant hematopoietic disorders.
PMID: 15481440
____________
If you were the careful sort of reader and actually looked at all the names of drugs in the table from Nature that I quoted above, you would have seen a familiar name: EGCG. This is our old friend, the green tea extract that has gained great popularity among cancer patients (Do You Like Drinking Green Tea?). As the Nature Review points out, EGCG is a potent DNA de-methylating agent. I chased down their reference, and the abstract as well as link to the full text of the article are given below.
Cancer research Article
Cancer Res. 2026 Nov 15;63(22):7563-70
Tea polyphenol (-)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines.
Fang MZ, Wang Y, Ai N, Hou Z, Sun Y, Lu H, Welsh W, Yang CS.
Department of Chemical Biology, Ernest Mario School of Pharmacy, Rutgers, The State University of New Jersey, 164 Frelinghuysen Road, Piscataway, NJ 08854-8020.
Hypermethylation of CpG islands in the promoter regions is an important mechanism to silence the expression of many important genes in cancer. The hypermethylation status is passed to the daughter cells through the methylation of the newly synthesized DNA strand by 5-cytosine DNA methyltransferase (DNMT). We report herein that (-)-epigallocatechin-3-gallate (EGCG), the major polyphenol from green tea, can inhibit DNMT activity and reactivate methylation-silenced genes in cancer cells. With nuclear extracts as the enzyme source and polydeoxyinosine-deoxycytosine as the substrate, EGCG dose-dependently inhibited DNMT activity, showing competitive inhibition with a K(i) of 6.89 microM. Studies with structural analogues of EGCG suggest the importance of D and B ring structures in the inhibitory activity. Molecular modeling studies also support this conclusion, and suggest that EGCG can form hydrogen bonds with Pro(1223), Glu(1265), Cys(1225), Ser(1229), and Arg(1309) in the catalytic pocket of DNMT. Treatment of human esophageal cancer KYSE 510 cells with 5-50 microM of EGCG for 12-144 h caused a concentration- and time-dependent reversal of hypermethylation of p16(INK4a), retinoic acid receptor beta (RARbeta), O(6)-methylguanine methyltransferase (MGMT), and human mutL homologue 1 (hMLH1) genes as determined by the appearance of the unmethylation-specific bands in PCR. This was accompanied by the expression of mRNA of these genes as determined by reverse transcription-PCR. The re-expression of RARbeta and hMLH1 proteins by EGCG was demonstrated by Western blot. Reactivation of some methylation-silenced genes by EGCG was also demonstrated in human colon cancer HT-29 cells, esophageal cancer KYSE 150 cells, and prostate cancer PC3 cells. The results demonstrate for the first time the inhibition of DNA methylation by a commonly consumed dietary constituent and suggest the potential use of EGCG for the prevention or reversal of related gene-silencing in the prevention of carcinogenesis.
PMID: 14633667
____________
I wonder what it will take for some one to come up with a clinical trial design that uses the combination of valproic acid (HDAC inhibitor) plus EGCG (de-methylating agent).
While the majority of CLL Topics members are from North America and Europe, we are an international organization with readers from all over the world. Once every little while I get wistful letters from patients who live in less affluent societies, or even people living in wealthy countries but who happen not to have medical insurance. I remember one such letter with great clarity. The letter said CLL Topics website is like a candy store, with all sorts of goodies displayed in the windows. But only the rich kids can get in to buy the expensive candy, the poor boy can only look from the outside, nose to the glass window pane. Drugs like Rituxan, Campath or even fludarabine are out of the reach of most patients living in poor countries, out of reach of even our neighbors if they do not have insurance. My friend went on to say the indolent nature of CLL is almost a curse in his case, he just wanted to get it over and done with. Without insurance to pay for it, each infusion, each trip to the doctor costs more than he can afford, and the constant infections mean he cannot get to work most days. Agitating for more money and resources for CLL patients does not help, not if there is no more money to be had. There is only so much pie, if one group gets a bigger slice some others have to go hungry. Economies can go into bankruptcy covering the sharply escalating healthcare costs, even rich countries like ours are going to face this problem in the years to come.
Finding ways to manage CLL clearly needs good science, good research, good medical breakthroughs. But it needs more than that, good science is not enough. We also need to address public policy and economic issues. Epigenetic therapy approaches we have discussed in this article are a bright ray of hope, they may provide some answers to these complex issues. Drugs such as valproic acid, EGCG, chlorambucil are all orally available, off-patent and cheap. No expensive visits to the doctor, no infusions that cost several thousand dollars a pop, no lost time from work. At the low doses that are thought to be effective in epigenetic therapy approaches, these are all drugs that have been proven to have low toxicity. A very big part of the overall dollar cost of CLL management is the cost of hospitalizations due to infections, as well as growth factor support for disease or therapy related anemia and neutropenia. Procrit and Neulasta are advertised on American TV, with glowing pictures of happy chemotherapy patients full of zest for life. Can you imagine what it feels like to be weighed down with deep fatigue, being sick all the time, because you do not have enough red blood cells or neutrophils, and these miracle drugs they are talking about are out of your reach?
I am all for state-of-the-art research, ever smarter drugs that will nail the CLL cells, and a list of supporting drugs such as Leukine, Neupogen, Neulasta, Procrit, etc., to take care of the incidental drop in blood counts. All of these and the newer antibiotics, anti-virals and anti-fungals to protect against infections cost an arm and a leg. While our best brains are working on the next breakthrough that will win Nobel Prizes and make billion dollar profits, don't you think that the less sexy, more readily available combinations should also be considered? Does the epigenetic approach of low dose combination of valproic acid (HDAC inhibition) + EGCG (de-methylation) + chlorambucil (a little gentle kick in the butt to encourage the CLL cells to die) have merit? I do not know. It surely has the virtue of being low cost, available and easy to administer, with the potential for low toxicity. As for its ability to give patients a decent quality of life and longer survival - that needs to be proven in clinical trials.
My question is this: where will be find our champions, the researchers that do not mind doing less than sexy clinical trials, and the money for funding clinical trials when there is no profit to be made in these drugs, and local oncologists that will recommend such combinations to their patients, even when there are no financial incentives from drug companies for using their products, no income from infusion room charges? I do not have answers to these questions, but I promise you I will keep looking.
 Enter Keywords: |
———
Disclaimer: The content of this website is intended for information only and is NOT meant to be medical advice. Please be sure to consult and follow the advice of your doctors on all medical matters.
Copyright Notice:
Copyright © 2026-2007 CLL Topics, Inc. All Rights Reserved.
All materials contained on this site are protected by United States copyright law and may not be reproduced, distributed, transmitted, displayed, published or broadcast without the prior written permission of CLL Topics, Inc. You may not alter or remove any trademark, copyright or other notice from copies of the content.
However, you may download and print material from CLLTopics.org exclusively for your personal, noncommercial use.
———
