 |
||||||||||
Date: July 20, 2026
by Chaya Venkat
Index Page for Related Articles:
HuMax-CD20
 I have been pondering this question as I have watched my old friend “Harvey” (Harvey Is Back!; Results from Genmab's HuMax-CD20 Phase II Clinical Trial) responding to his latest round of therapy with HuMax-CD20. Both Rituxan and HuMax-CD20 are monoclonal antibodies that target CD20, the marker present on mature B-cells — and none of the other immune system cell lines. Rituxan has been around for several years now, a very famous example of commercially successful monoclonal antibody technology. It has become the Goliath of the biotech industry, with revenues measured in billions of dollars. Compared to Rituxan, HuMax-CD20 is a new upstart. True, it has received fast-track status from the FDA for clinical trials in CLL but final approval to market the drug will depend on the success of the latest Phase-III clinical trial that has been launched. Will this David have any success in taking on the established Goliath of Rituxan? In this article I would like to review for you some of the differences between the two drugs, what makes them tick, why they seem to behave so differently in CLL patients.
I have been pondering this question as I have watched my old friend “Harvey” (Harvey Is Back!; Results from Genmab's HuMax-CD20 Phase II Clinical Trial) responding to his latest round of therapy with HuMax-CD20. Both Rituxan and HuMax-CD20 are monoclonal antibodies that target CD20, the marker present on mature B-cells — and none of the other immune system cell lines. Rituxan has been around for several years now, a very famous example of commercially successful monoclonal antibody technology. It has become the Goliath of the biotech industry, with revenues measured in billions of dollars. Compared to Rituxan, HuMax-CD20 is a new upstart. True, it has received fast-track status from the FDA for clinical trials in CLL but final approval to market the drug will depend on the success of the latest Phase-III clinical trial that has been launched. Will this David have any success in taking on the established Goliath of Rituxan? In this article I would like to review for you some of the differences between the two drugs, what makes them tick, why they seem to behave so differently in CLL patients.
The scientific name for HuMax-CD20 is ofatumumab - not something that rolls off the tongue easily, but a name you should remember nonetheless. This is going to be an important drug for CLL patients. I am willing to bet dollars to donuts on that.
Genmab has just announced the clinical trial on www.clinicaltrials.gov, with all the details. This is a multi-center trial, offered at 12 locations across the USA as well as many more across Europe. You can read all about it by clicking on the link below. I am pleased to see such a detailed listing of the inclusion and exclusion criteria, as well as contact phone numbers for each of the many sites where the trial is offered. This is not a “stealth trial”. Some of the best CLL expert centers are participating in this trial. In fact, the long list of investigators reads like a who's-who of CLL research, starting with study chairs Prof. Anders Österborg of the Karolinska Institute in Sweden and Prof. Bill Wierda of M. D. Anderson. Here is the link to the trial listing on the clinicaltrials.gov website: http://clinicaltrials.gov/show/NCT00349349
Here are a few highlights of the trial:
This is a pretty gutsy trial. The inclusion criteria guarantee that they are going to be dealing with a very tough crowd, patients who have flunked both fludarabine and Campath, the two most potent drugs we have in our arsenal. Just for comparison, single agent Rituxan would have next to no response in patients with this profile. Chances are high that a substantial percentage of these patients are going to have the dreaded p53 (17p) deletion, since this aggressive clone is often the end result in fludarabine refractory patients. Earlier work has suggested that Rituxan cannot handle p53 deleted cases (Byrd, et al.) but Campath is supposed to be able to work in spite of this chromosomal defect. The target profile of Campath relapse suggests patients recruited for this HuMax-CD20 trial are likely to have bulky lymph nodes, something that stymies even Campath. All in all, this is about as tough as it gets in terms of the chosen patient population.
Perhaps this is one of the few scenarios where we may not need a control group to judge the merits of this new drug, since patients with this profile have few good options and they would not be expected to respond to anything other than massively aggressive chemo-immunotherapy combinations. If Genmab can pull off decent responses in this clinical trial without massive adverse effects, I would think they have proved their case and the FDA approval to market the drug commercially might be given in real quick time. If it works well in such late stage patients, that is good news for all of us, since it is likely to work that much better in less advanced cases. Additionally, as we saw in the case of our favorite hypothetical patient "Harvey", this new anti-CD20 monoclonal may become the lifeline for patients who develop serious hypersensitivity reactions to Rituxan.

Much of my discussion below is gathered from two pivotal articles, their abstracts are given below. Do not hesitate to write to us if you want help in locating the full texts. However, I must warn you neither of these is easy reading material. You have to be a bit of a glutton for technical detail and jargon to get through them. I enjoyed reading both of them immensely. I had the distinct pleasure of meeting Dr. Martin Glennie, one of the authors that contributed a great deal to both of these articles. I am not sure Martin will speak to me again after he reads my low-brow translation of their sophisticated science.
Blood. 2026 Sep 15;104(6):1793-800. Epub 2026 Jun 1.
Characterization of new human CD20 monoclonal antibodies with potent cytolytic activity against non-Hodgkin lymphomas.
Teeling JL, French RR, Cragg MS, van den Brakel J, Pluyter M, Huang H, Chan C, Parren PW, Hack CE, Dechant M, Valerius T, van de Winkel JG, Glennie MJ.
Genmab, Yalelaan 60, 3584 CM Utrecht, The Netherlands.
Despite the rapid and widespread integration of chimeric CD20 monoclonal antibody (mAb), rituximab, into the management of non-Hodgkin lymphoma, its efficacy remains variable and often modest when used as a single agent. To develop more potent reagents, human immunoglobulin transgenic mice were used to generate a panel of immunoglobulin G1kappa (IgG1kappa) CD20 mAbs. All reagents bound strongly to CD20(+) cells and recruited mononuclear cells for the lysis of malignant B cells. However, 2 mAbs, 2F2 and 7D8, were exceptionally active in complement-dependent cytotoxicity (CDC), being able to lyse a range of rituximab-resistant targets, such as CD20-low chronic lymphocytic leukemia (CLL), in the presence of human plasma or unfractionated blood. Further analysis showed that 2F2 and 7D8, like rituximab, redistributed CD20 into Triton X-100-insoluble regions of the plasma membrane, but that they had markedly slower off-rates. To determine whether off-rate influenced CDC, a non-complement activating F(ab')(2) antihuman kappa reagent was used. This reagent markedly slowed the off-rate of rituximab and increased its CDC activity to that of 2F2 and 7D8. Thus, with increasing evidence that mAb therapeutic activity in vivo depends on complement activation, these new CD20 reagents with their slow off-rates and increased potency in CDC hold considerable promise for improved clinical activity.
PMID: 15172969
____________
J Immunol. 2026 Jul 1;177(1):362-71.
The biological activity of human CD20 monoclonal antibodies is linked to unique epitopes on CD20.
Teeling JL, Mackus WJ, Wiegman LJ, van den Brakel JH, Beers SA, French RR, van Meerten T, Ebeling S, Vink T, Slootstra JW, Parren PW, Glennie MJ, van de Winkel JG.
Genmab, Utrecht, The Netherlands.
We have previously defined a panel of fully human CD20 mAb. Most of these were unexpectedly efficient in their ability to recruit C1q to the surface of CD20-positive cells and mediate tumor lysis via activation of the classical pathway of complement. This complement-dependent cytotoxicity (CDC) potency appeared to relate to the unusually slow off-rate of these human Abs. However, we now present epitope-mapping data, which indicates that all human mAb bind a novel region of CD20 that may influence CDC potency. Epitope mapping, using both mutagenesis studies and overlapping 15-mer peptides of the extracellular loops of CD20, defined the amino acids required for binding by an extensive panel of mouse and human mAb. Binding by rituximab and mouse CD20 mAb, had an absolute requirement for alanine and proline at positions 170 and 172, respectively, within the large extracellular loop of CD20. Surprisingly, however, all of the human CD20 mAb recognize a completely novel epitope located N-terminally of this motif, also including the small extracellular loop of CD20. Thus, although off-rate may influence biological activity of mAb, another critical factor for determining CDC potency by CD20 mAb appears to be the region of the target molecule they recognize. We conclude that recognition of the novel epitope cooperates with slow off-rate in determining the activity of CD20 Ab in activation of complement and induction of tumor cell lysis.
PMID: 16785532
____________
We discussed in a prior article (Sons of Rituxan and Campath) the number of ways in which monoclonal antibodies like Rituxan can kill B-cells that carry the CD20 marker. I will not go into the full details here, just enough to jog your memory. In a nutshell, once the monoclonals have latched on to the CD20 markers on the target cells, killing can happen by three different mechanisms: CDC, ADCC and direct cell kill. We examine these mechanisms in the following sections.
“Complement” refers to a cascade of inter-related proteins that are important in defending our bodies against a wide range of bacteria. Over our lifetimes, our bodies have learned to recognize certain markers (“antigens”) on bacteria and developed antibodies (immunoglobulins) that are specific to each of these antigens. As soon as one of these known bacteria is seen, the antigens on the surface of the bacteria are quickly tagged by the corresponding antibody molecules circulating in our blood. The presence of these antigen-antibody pairs on the surface of the microbe are like neon advertising signs that attract complement proteins to swoop down and kill the cell.
I hope you can see the parallel between this scenario and what happens when we treat a CLL patient with a man-made anti-CD20 monoclonal antibody. Here the hapless B-cell is in the role of the bacterium, the CD20 marker it carries is the antigen and the man-made monoclonal antibody drug is the synthetic immunoglobulin that mates with the CD20 marker. Presto, we have achieved the all important antigen-antibody pair on the surface of the B-cell and that should cause rapid coating of the cell with complement proteins (“opsonization”) and thereby cause the cell to die a quick death.
Not quite. Complement is such a powerful killer that the body has to have some safeguards in place to protect itself from the kind of over-exuberant and uncontrolled killing rampage that will do more harm than good. Just the odd one or two pairs of antigen-antibody are not enough to cause complement attack. Real bacteria have literally tens of thousands of antigens on their surface and our bodies have evolved to have complement react only when there is a sufficiently large number of antigen-antibody pairs on the surface of the bacteria. A single neon advertising sign will not do — it has to be a Vegas-style ostentatious overkill before complement will take notice.
This is one of the major differences between CLL and non-Hodgkin’s lymphoma. CD20 expression is dim on CLL cells, whereas it is bright on NHL cells. There may be only 10,000 – 20,000 or so CD20 markers per CLL cell, while there can be many times more than that on each NHL cell. Net result: it is harder to get enough antigen-antibody pairs on the surface of each CLL cell to initiate complement-mediated cell kill. NHL cells do not have this problem, since they are richly covered with CD20 markers — when this profusion of CD20 markers is tagged by Rituxan, there is a sufficient density of antigen-antibody pairs that complement gets to work and kills the cell.
Even so, things might be a little different if Rituxan made use of all the available CD20 markers on CLL cells. It so happens that Rituxan is only loosely bound to the CD20 marker. Imagine a velcro fastener that has seen better times. Suppose the two pieces of the velcro sort of stick on and off and half the time the two pieces are not bound together at all. In other words, we do not have a superabundance of CD20 on CLL cells to begin with and a good proportion of them may not be bound by Rituxan at any given time. Net result: there are not enough CD20-Rituxan pairs on each cell to attract complement and there is precious little cell kill by this important pathway.
This is one of the areas where HuMax-CD20 is different from Rituxan. It binds to a different part of the CD20 marker — and it binds to it a whole lot better. Like a new piece of velcro, the HuMax antibody stays firmly bound to the CD20 markers on B-cells, with a lot less of ‘off’ time. Net result: it makes the most of the situation and can get a higher number of antigen-antibody pairs going per cell, more neon lights flashing to attract the attention of the lethal complement proteins.
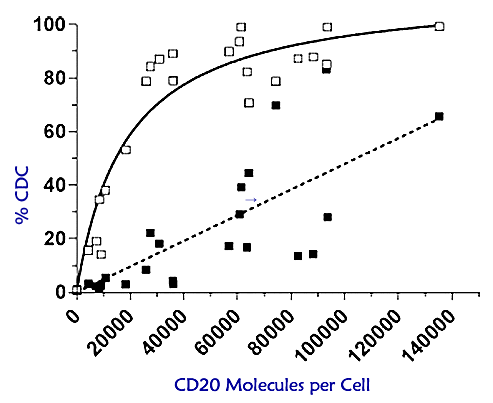 |
Biological Activity of Human CD20 Monoclonal Antibodies
Teeling, et al., Genmab.
The graph above is from the second article. As you can see, the percentage of cell kill by complement (“%CDC”) increases as the number of CD20 molecules per cell increases. The dashed line is for Rituxan, the solid line is for HuMax-CD20. At about 10K – 20K of CD20 markers per cell (the range present in most CLL patients), the percentage of cells killed by CDC pathway is a puny 5% or less for Rituxan. It is many times more than that for HuMax-CD20, a hefty 30-50%. If you are one of the fortunate CLL patients with an even higher expression of CD20 (a “medium” expression versus the “dim” expression common for most CLL patients), say in the 20K -30K region of CD20 per B-cell, you can expect to see even higher levels of cell kill via the complement-dependent pathway using HuMax-CD20.
Rituxan and HuMax-CD20 are “Y” shaped molecules, capable of grabbing onto the CD20 markers on CLL cells with the two arms of the “Y”. As we have seen above, it seems HuMax-CD20 does a better job of grabbing and holding on to the CD20 markers.
But the tail of the “Y” is also important. Once CD20-Rituxan (or HuMax) pairs have been formed on the surface of the CLL cell, the many tails of the “Y” hanging out there flapping in the breeze are incredibly attractive and sexy to any immune system effector cells passing by. NK cells, macrophages, neutrophils and T-cells are drawn to these “tails” (more properly called “Fc” regions of the antibodies) and mate with them. The result of this is that a death signal is passed to the hapless CLL cell, inviting it to commit suicide. This may be sufficient incentive for many CLL cells to do as they are told and die quietly. Unfortunately, CLL cells that have defects in the p53 (17p) gene may also have defects in their suicide machinery and may not pay much attention. This is why CLL clones with p53 defects are so hard to kill and why patients with this chromosomal defect have poor responses to so many drugs.
As we discussed above, since CDC does not work so well for Rituxan, the ADCC pathway is crucial for cell kill using this drug. Unfortunately, the ADCC mechanism requires the cooperation of NK cells, macrophages, neutropils and T-cells. The function of these killer cells is often compromised in late stage CLL patients, often as a result of prior chemotherapy. This may explain why Rituxan as a single agent works to some degree in chemo-naïve patients, in whom the rest of the immune system is still in pretty good shape and therefore ADCC is able to work reasonably well, but the drug has poor response rates in previously treated patients. In patients who have been through prior therapy, chances are the effector cells are not working too well anymore and therefore this important pathway for cell kill is also compromised. The situation becomes even more difficult if the patient has a CLL clone with p53 defects, since this type of CLL cell is resistant to the ADCC request to commit suicide. HuMax-CD20, on the other hand, may not face the same issues since it is able to more effectively use the CDC cell kill pathway — and that pathway does not depend on the health and well being of NK cells, macrophages, neutrophils and T-cells.
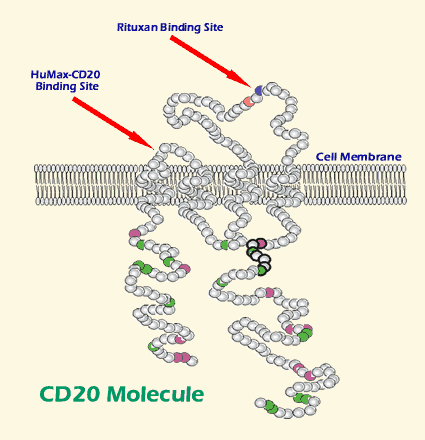 There is one more little detail that may explain the differences between Rituxan and HuMax-CD20. When the polite ADCC request to commit suicide is not enough, NK cells, macrophages, neutrophils and T-cells also have a much more blunt way of killing the target cell. They can build little tubes that make a hole and bore into the target CLL cell, then pump in poisonous granules that will kill the CLL cell. (We discussed this approach of perforin- and granzyme-based cell kill in a prior article Killer T-cells). The HuMax-CD20 binds tightly to a region of the CD20 marker that is close to the surface of the CLL cell. Rituxan, on the other hand, binds to a more distant region of the CD20 marker, one that keeps the antibody from getting up close and tight against the CLL cell surface. Well, there is in fact some research that suggests the tight binding of HuMax-CD20 close to the surface of the B-cell makes the process of poisoning the CLL cell by effector cells easier, since the perforin tube has a shorter distance to travel before it can penetrate the surface of the CLL cell. The more distant binding site of Rituxan means the effector cells have to build longer perforin tubes before they can reach the CLL cell surface and the effector cells therefore have a harder time of pumping in the poison.
There is one more little detail that may explain the differences between Rituxan and HuMax-CD20. When the polite ADCC request to commit suicide is not enough, NK cells, macrophages, neutrophils and T-cells also have a much more blunt way of killing the target cell. They can build little tubes that make a hole and bore into the target CLL cell, then pump in poisonous granules that will kill the CLL cell. (We discussed this approach of perforin- and granzyme-based cell kill in a prior article Killer T-cells). The HuMax-CD20 binds tightly to a region of the CD20 marker that is close to the surface of the CLL cell. Rituxan, on the other hand, binds to a more distant region of the CD20 marker, one that keeps the antibody from getting up close and tight against the CLL cell surface. Well, there is in fact some research that suggests the tight binding of HuMax-CD20 close to the surface of the B-cell makes the process of poisoning the CLL cell by effector cells easier, since the perforin tube has a shorter distance to travel before it can penetrate the surface of the CLL cell. The more distant binding site of Rituxan means the effector cells have to build longer perforin tubes before they can reach the CLL cell surface and the effector cells therefore have a harder time of pumping in the poison.
The third mechanism by which antibody drugs can kill the cells they tag does not require the help of complement (CDC) or effector cells (ADCC). The command to die is given directly by the antibody binding to its target marker. Presumably both Rituxan and HuMax-CD20 do a certain amount of direct cell kill, but I get the impression this is not a major pathway for either of them. There is another CD20 monoclonal antibody in the wings, a type of monoclonal called B1, that has very potent ability to kill the cell it is locked on to, with no dependence on complement or effector cells. This “B1-bomber” has shown remarkably higher cell kill rates than either of Rituxan or HuMax-CD20 in laboratory studies. Unfortunately it will be a while before we can expect to see it in human clinical trials.
Rituxan is a chimeric monoclonal antibody, which means a part of the molecule is based on mouse protein. HuMax-CD20, on the other hand, is fully humanized and has no mouse components. This may explain potential differences in the toxicity of the two drugs. Recently, we have reported delayed onset hypersensitivity reactions developed by some patients who have been through Rituxan therapy (Rituxan Roadblock). It is thought the hypersensitivity could be due to the mouse components in Rituxan — and if so, similar problems may not be an issue for HuMax-CD20.
However, the fact that HuMax-CD20 invokes a stronger response from complement as we discussed above may mean that there could be more infusion-related adverse effects. Our favorite hypothetical patient “Harvey” had an attack of hives (“urticaria”) within ten minutes of starting his first HuMax-CD20 infusion (Harvey Is Back!). The infusion was stopped and the rash went away in a half an hour or so, allowing re-start of the infusion. However, this type of allergy response can be a lot more pronounced in other patients. It is important that the infusion is started very slowly and all the prescribed pre-medications (anti-histamines, for example) are administered as recommended. You will notice that the clinical trial design calls for a first infusion of only 300 mg administered over 6 ½ hours while the remaining 11 infusions are 2,000 mg each. This is important since it allows the first 300 mg to be given very, very slowly.
Those of you who have followed our Campath articles closely may recall that there were very significant infusion related side effects with intravenous Campath administration. Most of these problems went away when the method of administering the drug was changed from intravenous infusion to a sub-cutaneous injection. The sub-q approach allows the drug to leach into the body slowly over a long period of time, and this was sufficient to take care of the problems. Unfortunately, unlike Campath where drug dosage is typically 30 mg or so, HuMax-CD20 drug dosages are way too large for subcutaneous injection. It means you should be prepared for mind-numbingly slow drug infusion, and a very long day in that infusion chair when you are getting HuMax-CD20.
“Harvey” noticed that after completing his earlier rounds of Rituxan therapy he had often ended with low neutrophil counts, almost borderline neutropenic levels after the several weeks of Rituxan therapy. A low level of neutrophils carries the risk of picking up some opportunistic infection or the other. Harvey wondered if this was due to his neutrophils all getting chewed up as they had to work extra hard as effector cells in the ADCC duty of killing Rituxan-tagged CLL cells. By contrast, with HuMax-CD20 his neutrophils counts were in the pink after finishing four weeks of HuMax-CD20 therapy and continue to do very well 2 months later. Delayed-onset neutropenia was a concern in Rituxan therapy, it will be interesting to see how Harvey’s neutrophil counts hold up over the months to come.
Some of the mechanisms and differences between Rituxan and HuMax-CD20 discussed above are still in the realm of ongoing research. But I thought they explain, at least partly, the choices Genmab has made in their clinical trial design. Mind you, this is purely my understanding of why I think they have decided to do what they are doing. They have not explained their thinking to me.
This clinical trial uses massive amounts of HuMax-CD20, a total of 22,300 mg of the monoclonal antibody per patient over the 6 months. This raises a few questions in my mind.
Bottom line, there are reasons for hoping that HuMax-CD20 can achieve good CDC cell kill even with low the CD20 antigen density characteristic of most CLL patients — and get more ADCC cell kill even in fludarabine refractory and potentially p53 defective cases. The results of this Phase III clinical trial will go a long way to show if this is indeed the case. I am keeping my fingers crossed on this one — it is about time we got some new and better choices for our “Bucket C” old timers who have already been through the chemo wars. Until now, Campath (and perhaps high dose steroids) is the one drug that has shown promising efficacy in these patients but at the risk of massive T-cell depletion and the resulting risk of opportunistic infections. A CD20-targeting monoclonal antibody that works better than Rituxan (without the immune suppression of Campath) for this segment of our population will be welcome news. And if it works well for them, I am sure there will be clinical trials addressing other patient groups for whom it may be even more effective. We will all have gained a powerful new weapon in our arsenal, one that promises better response rates with less collateral damage than the alternatives available today.
 Enter Keywords: |
———
Disclaimer: The content of this website is intended for information only and is NOT meant to be medical advice. Please be sure to consult and follow the advice of your doctors on all medical matters.
Copyright Notice:
Copyright © 2026-2007 CLL Topics, Inc. All Rights Reserved.
All materials contained on this site are protected by United States copyright law and may not be reproduced, distributed, transmitted, displayed, published or broadcast without the prior written permission of CLL Topics, Inc. You may not alter or remove any trademark, copyright or other notice from copies of the content.
However, you may download and print material from CLLTopics.org exclusively for your personal, noncommercial use.
———
