 |
||||||||||
Date: May 17, 2026
by Chaya Venkat

This article is about a recent and extremely important finding in our understanding of CLL and how to make the right therapy decisions for patients with potentially very different prognoses. This review is based on two very important papers — one by a group headed by Drs. Hamblin and another from Drs. Damle, et al. You can read the actual papers by using the references I cite in the text.
Before we get to the subject matter however, we need to understand the life cycle of a B-lymphocyte.
The bone marrow is the source of all blood cells, including the cells of the immune system called B-lymphocytes and T-lymphocytes. Both of these cell lines originate from the same precursor stem cells.
Once a particular lymphocyte cell is committed to become a T-cell, it migrates from the bone marrow to the Thymus, (that is why they are called "T-cells", T for Thymus), where it completes its education on how to function as an effective T-cell. Some of the T-cells hang around in the thymus, while others circulate within the peripheral blood and lymphatic system.
Lymphocytes that are destined to be B-cells do not go to the thymus. They do much of their maturation in the bone marrow, then travel through the peripheral blood to the lymphatic system. At this point the B-cell is called a "naïve" B-cell, because it has not yet been programmed to attack any specific antigen. (Any substance capable of triggering an immune response is known as an antigen. An antigen can be a bacterium or a virus, or even a portion or product of one of these organisms. Tissues or cells from another individual also act as antigens; that's why transplanted tissues are rejected as foreign).
The necessary programming of the B-cells to do their job of fighting antigens happens in the lymph nodes, which are small, bean-shaped structures that are laced throughout the body along the lymphatic routes. Lymph nodes contain specialized compartments called "germinal centers" where immune cells congregate, and where they can encounter antigens. Once exposed to a specific antigen, a particular B-cell is programmed to fight just that antigen.
This is done by mutating the gene sequence within the B-cell that controls the formation of immunoglobulins (Ig) on the surface of the B-cell. Immunoglobulin molecules are "Y" shaped structures, anchored at the stem, with the tips of the "Y" acting as the business end. The job of the genes controlling its formation is to see that the tips (Variable Region) immunoglobulin molecule have exactly right shaped pincers for matching the antigen. This is what is meant by the term "IgVH mutation", mutation of the gene controlling the shape of the Variable region of the Heavy chain of the Immunoglobulin. This type of mutation is also called "somatic mutation". Once this necessary mutation has taken place, the B-cell remembers this piece of programming, and becomes what is called a "memory cell". In future, all its daughters will remember and reflect this programming. They will all have the same exact shape to the tips of their Ig, and they will all fight only the specific antigen their mother cell had been programmed to fight. To recap, B-cells originate from stem cells in the bone marrow. Prior to completing their programming ("IgVH gene mutation" or "somatic mutation") in the lymph nodes, they are called "naïve B-cells". After exposure to an antigen in the lymph nodes, causing the necessary IgVH gene mutation, they and all their daughters become primed to fight that specific antigen. They are now called "memory B-cells".
One of the remarkable things about the immune system is its ability to recognize many millions of distinctive antigen molecules, and to respond by producing memory B-cells that can match and be ready to counteract each one of these individual antigens. The B-cell's inherent capacity to rearrange and mutate its genes that control immunoglobulin formation is critical for making memory cells capable of attacking the millions of antigens out there. However, this capacity also introduces risk since the processes of immunoglobulin gene rearrangement and mutation can go awry, resulting in unintentional rearrangement or dangerous mutation of genes involved in vital cell functions. In addition to this high mutation risk, B-cells must have an innate capability to undergo high rates of proliferation at various stages of differentiation or in response to antigen. This ability to mutate readily, and high proliferation rates at certain times sets the stage for potential cancer initiation.
The undesired and dangerous mutation of B-cells (do not confuse this with the necessary and required "IgVH gene mutation" or "somatic mutation") can happen at any point in their life cycle. Researchers seem to have identified two distinct groups of patients with CLL, those whose cancer started with a single "naïve" B-cell that underwent an undesired gene mutation prior to its entry into the germinal center in the lymph node, and the second set of patients whose cancer started with a "memory" B-cell that went bad, after its stay within the germinal center. As we discussed above, the first group will have clonal B-CLL cells that do not have IgVH gene mutation, while the second group will have clonal population of B-CLL cells that will show IgVH gene mutation.
In the article below, Terry Hamblin, et. al., discuss the very different prognosis for the two groups of patients. The whole article is very worth reading, but I have extracted the two graphs that show the results. As you can see, the patients whose CLL started with a "memory" cell that went bad (i.e., whose CLL cells exhibit IgVH gene mutation) live a lot longer than those whose cancer started with a "naïve" B-cell (i.e., patients whose CLL cells do not exhibit IgVH gene mutation).
Link to full text article:Blood Journal Article
Unmutated Ig VH Genes Are Associated With a More Aggressive Form of Chronic Lymphocytic Leukemia.
By Terry J. Hamblin, Zadie Davis, Anne Gardiner, David G. Oscier, and Freda K. Stevenson.
Dr. Hamblin's group examined the VH genes of 84 patients with classical B-cell CLL. 38 patients (45.2%) had IgVH genes with greater than 98% of their sequence identical to the germline gene, (i.e., they had unmutated IgVH genes), the remaining 46 cases (54.8%) showed evidence of IgVH somatic mutation, dividing the patients into 2 subsets. The two groups had vastly different survival characteristics.
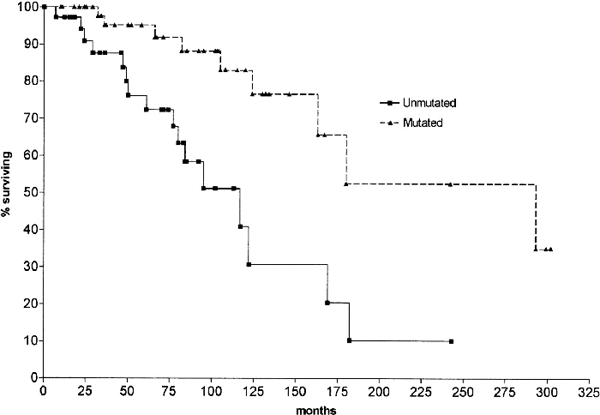
Survival curves comparing CLL patients with mutated |
Median survival for unmutated CLL: 117 months; median survival for mutated CLL: 293 months. (Another way of saying the same thing — 50% of the patients with unmutated IgVH genes died by 117 months, while it took 293 months for 50% of the patients with mutated IgVH to die). The same pattern was obtained when comparing only early stage (Binet stage A, or roughly, Rai stage I/II) patients with or without IgVH gene mutation.
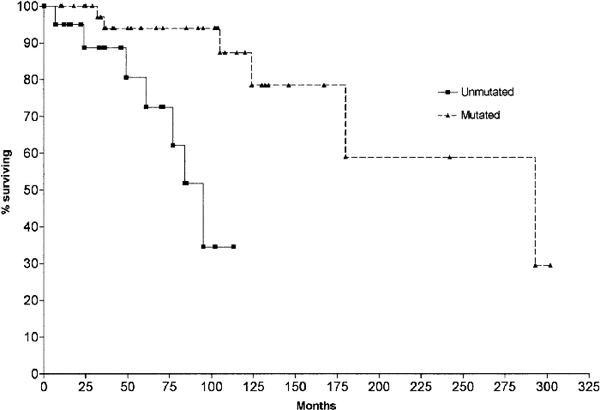
Survival curve comparing stage A CLL patients with mutated |
Median survival for unmutated CLL: 95 months; median survival for mutated CLL: 293 months.
Clearly, if you are an early stage CLL patient, say about 50 years old, and you have IgVH gene mutation detected in your CLL cells, you have an even chance of living another 24.4 years, perhaps die of old age at 75. Not a bad prognosis, and you and your family will breathe a sigh of relief. If, however, you fall into the other camp, and you have an even chance of seeing only about 8 years, you may want to explore the therapy options open to you. At the end of this paper is a reference to a case based study by a blue ribbon panel of CLL experts. The one opinion they all agreed upon was this: getting a good fix on prognosis is important, so that the therapy can be tailored for that particular prognosis. It is dangerous to watch and wait too long if the indications are of an aggressive disease, and it is unnecessary to expose the patient to the rigors of chemotherapy if the prognosis says he/she is likely to die of old age!
Sounds reasonable we should all go out and get our IgVH gene mutation status ascertained. Not so fast. This is something that only the best of the research labs are able to do, it is an expensive and time consuming process, and not too many oncologists even know what it takes to get it done. So what is one to do?
The article below by Dr. Damle, et. al. offers an alternative. His group found that there is a strong correlation between IgVH gene mutation status and the level of CD38 expressed by the CLL cells. High CD38 (more than 30% on CLL cells) correlated with unmutated IgVH genes, and low level of CD38 tracked mutated IgVH genes. Now, it is not so hard to measure the CD38, first by making sure you got the CLL cells only in your population (CD19 and CD5 positive cells), then checking to see how many of them had CD38 as well. It is not a trivial job, but most labs than can handle quantitative flow cytometry can do this, certainly all of the consortium specialist hospitals. The whole paper is worth reading, but again I have extracted the really critical results in the graphs below.
Link for full text article: Blood Journal Article
By Rajendra N. Damle, Tarun Wasil, Franco Fais, Fabio Ghiotto, Angelo Valetto, Steven L. Allen, Aby Buchbinder, Daniel Budman, Klaus Dittmar, Jonathan Kolitz, Stuart M. Lichtman, Philip Schulman, Vincent P. Vinciguerra, Kanti R. Rai, Manlio Ferrarini, and Nicholas Chiorazzi.
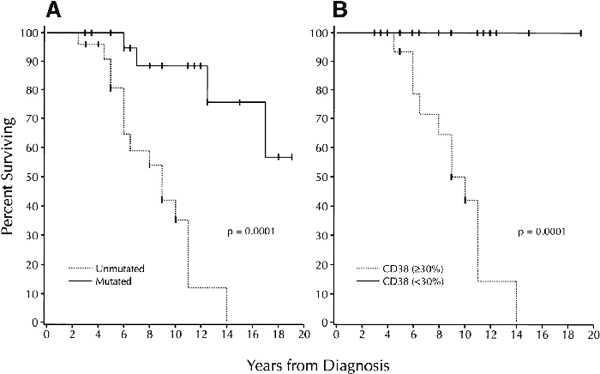
Survival based on V gene mutation status and CD38 expression |
(A) survival based on the absence or presence of significant numbers (greater than 2%) of IgVH gene mutations. Median survival of unmutated group: 9 years; median survival of mutated group not yet reached;
(B) survival based on the detection of more than 30%,or less than 30% CD38+ B-CLL cells. Median survival of the more than 30% CD38+ group: 10 years; median survival of the less than 30% CD38+ group: not yet reached.
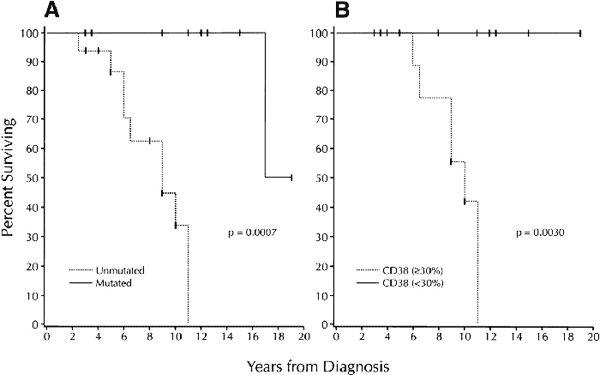
Survival based on V gene mutation status and CD38 expression |
(A) plot comparing V gene mutation status with survival. Median survival of the unmutated group: 9 years; median survival of the mutated group: 17 years.
(B) plot comparing more than 30%,or less than 30% CD38+ B-CLL cells with survival. Median survival of the more than 30% CD38+ group: 10 years; median survival of the less than 30% CD38+ group: not reached.
It is clear that CD38 is likely to be a useful prognostic indicator. There have been a flurry of papers on both IgVH gene mutation status and CD38 levels in CLL cells since the publication of these two seminal papers. All in all, the verdict seems to be that both are important indicators, but the jury is not quite in yet on whether one tracks the other, or the two are two independent indicators.
http://newscenter.cancer.gov/cancertopics/understandingcancer/immunesystem - Excellent tutorial by the National Cancer Institute on you immune system and how it works. Very readable, user-friendly and I strongly recommend you browse through it. A picture says a thousand words, and this site has excellent graphics.
ASH Education Book Article - Case based study by panel of experts, on the value of prognostic information in making therapeutic choices.
 Enter Keywords: |
———
Disclaimer: The content of this website is intended for information only and is NOT meant to be medical advice. Please be sure to consult and follow the advice of your doctors on all medical matters.
Copyright Notice:
Copyright © 2026-2007 CLL Topics, Inc. All Rights Reserved.
All materials contained on this site are protected by United States copyright law and may not be reproduced, distributed, transmitted, displayed, published or broadcast without the prior written permission of CLL Topics, Inc. You may not alter or remove any trademark, copyright or other notice from copies of the content.
However, you may download and print material from CLLTopics.org exclusively for your personal, noncommercial use.
———
