 |
||||||||||
Date: September 14, 2026
by Chaya Venkat
Related Articles: What Type of CLL Do You Have?
Prognosis at Diagnosis
IgVH Gene Mutation Status and CD38 Expression
Two prognostic indicators have become the focus of much discussion in recent months, ZAP-70 and IgVH mutation status. These two indicators, in combination with FISH analysis to pinpoint the chromosomal aberration that is at the root of the CLL, go a long way toward defining the risk category of the patient in question. We have discussed both ZAP-70 and IgVH in prior articles (What Type of CLL Do You Have?, Prognosis at Diagnosis, Prognostic Indicators).
But to tell the truth they are not the easiest concepts to understand. If you are like me, even if you actually understand the complicated jargon when you read the article, stuff like this has a way of evaporating out of your mind in a week or two, and then it is back to scratching your head the next time you see the acronyms. Several new articles have just been published that makes it worth our while to revisit this topic. For the first time, I see glimmers of hope that our understanding is reaching a point where therapy breakthroughs are within the time-frame of today's patients.
The abstract below is from a very recent issue of the New England Journal of Medicine, authored by all of the experts from the CLL Research Consortium. I will summarize the highlights for you, but if you want to read the full text of the article for yourself, or make a present of a hard copy of this important article to your local oncologist, write to us and we will help you locate it.
If you have been following prior discussions on these two prognostic indicators, you know that low levels of ZAP-70 and mutated IgVH genes spell good prognosis, whereas high ZAP-70 expression and unmutated IgVH genes spell trouble. The paper describes 307 CLL patients who were tested for ZAP-70 levels as well as the mutation status of their IgVH gene. This is a statistically large sample of patients, and the results make it clear that there is strong (but by no means perfect) correlation between ZAP-70 IgVH gene mutation status. The table below gives the details. As you can see, not all IgVH mutated cases are ZAP-70 negative and not all unmutated cases are ZAP-70 positive. For a significant percent of the patients studied the two prognostic indicators do not agree, 71 out of the 307 patients have conflicting indications. That is not a small number!
| Prognostic Indicator | IgVH Unmutated | IgVH Mutated |
| ZAP-70 Positive | 117 | 24 |
| ZAP-70 Negative | 47 | 119 |
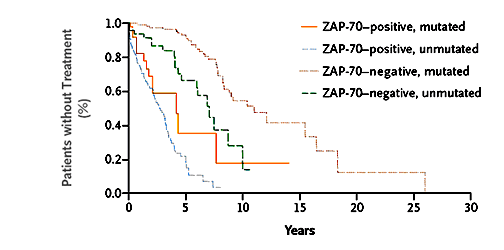
Patients Without Treatment
N Engl J Med. 2026 Aug 26;351(9):893-901
The graph above makes the crucial point. The authors point out that in the ZAP-70 positive patients, the median time from diagnosis to when therapy was needed was roughly the same, independent of whether they were IgVH mutated or not. It looks like when there is a difference of opinion between the two indicators, ZAP-70 trumps IgVH gene mutation status. The bottom line conclusion by this panel of CLL experts is quite clear: "Although the presence of an unmutated IgV(H) gene is strongly associated with the expression of ZAP-70, ZAP-70 is a stronger predictor of the need for treatment in B-cell CLL". Moral of the story, if you have managed to find your IgVH gene mutation status, you might want to finish the job and get your ZAP-70 test done as well, just in case you are one of those patients who have discordant results on these two prognostic indicators.
As we learn more about the molecular basis of CLL, it is rapidly becoming clear that CLL prognosis depends to a great deal on the ability of the cancer cell to interact with its surroundings. Cancer cells are blind, they cannot really "see" or "think", they live, proliferate or die depending on their ability to receive and transmit signals to the surrounding cells. The B-cell receptor (BCR) is one of the most important "data ports", a mechanism through which the cell receives signals to survive and proliferate. As the second abstract below points out, ZAP-70 is part of the BCR mechanism, a high level of ZAP-70 indicate a CLL cell that is only too happy to receive signals through this port. In general, IgVH unmutated cells are more likely to hear the message whereas cells with mutated IgVH genes have unresponsive BCR ports. That is why, in general, IgVH unmutated CLL is more aggressive and proliferates faster. However, there are special circumstances where even cells with mutated IgVH are responsive to antigen activation, and in these cases the ZAP-70 is positive reflecting the BCR responsiveness of the CLL cell in question. (To get a slightly more risqué analogy explaining these concepts, you might want to read the next article on the continuing saga of Harvey, our hypothetical Bucket C and Round-Headed Kid).
The good news is that ZAP-70 protein levels are likely to be much easier to measure, compared to determining IgVH gene mutation status. But there is work to be done before ZAP-70 becomes a prognostic test that delivers its value to CLL patients. Right now, there are no standardized procedures, no consensus across the board on appropriate cut-off points to define ZAP-70 positive and negative levels, and outside of top rated research institutions the validity of the results or their interpretation is suspect. We badly need some reputable organization such as the NCI, CLL Research Consortium, or highly regarded cancer centers such as the Mayo Clinic, Dana Farber, M. D. Anderson and the like to take the lead in defining the parameters of this test.
Here is a pop quiz, just to find out how many of you have actually read this long-winded article up to this point: how many of you think CLL Topics as a patient group should become pro-active in getting standardized and readily available ZAP-70 testing for all CLL patients, something that is quality controlled to yield meaningful results, that is within our reach in terms of logistics, and a test whose cost is covered by the major insurance companies? Write and let your opinion count. We have a lot more leverage working as a group than we do an individual patients. You know our address,  .
.
N Engl J Med. 2026 Aug 26;351(9):893-901.
ZAP-70 compared with immunoglobulin heavy-chain gene mutation status as a predictor of disease progression in chronic lymphocytic leukemia.
Rassenti LZ, Huynh L, Toy TL, Chen L, Keating MJ, Gribben JG, Neuberg DS, Flinn IW, Rai KR, Byrd JC, Kay NE, Greaves A, Weiss A, Kipps TJ.
Chronic Lymphocytic Leukemia Research Consortium, University of California, San Diego, La Jolla
BACKGROUND: The course of chronic lymphocytic leukemia (CLL) is variable. In aggressive disease, the CLL cells usually express an unmutated immunoglobulin heavy-chain variable-region gene (IgV(H)) and the 70-kD zeta-associated protein (ZAP-70), whereas in indolent disease, the CLL cells usually express mutated IgV(H) but lack expression of ZAP-70.
METHODS: We evaluated the CLL B cells from 307 patients with CLL for ZAP-70 and mutations in the rearranged IgV(H) gene. We then investigated the association between the results and the time from diagnosis to initial therapy.
RESULTS: We found that ZAP-70 was expressed above a defined threshold level in 117 of the 164 patients with an unmutated IgV(H) gene (71 percent), but in only 24 of the 143 patients with a mutated IgV(H) gene (17 percent, P<0.001). Among the patients with ZAP-70-positive CLL cells, the median time from diagnosis to initial therapy in those who had an unmutated IgV(H) gene (2.8 years) was not significantly different from the median time in those who had a mutated IgV(H) gene (4.2 years, P=0.07). However, the median time from diagnosis to initial treatment in each of these groups was significantly shorter than the time in patients with ZAP-70-negative CLL cells who had either mutated or unmutated IgV(H) genes (P<0.001). The median time from diagnosis to initial therapy among patients who did not have ZAP-70 was 11.0 years in those with a mutated IgV(H) gene and 7.1 years in those with an unmutated IgV(H) gene (P<0.001).
CONCLUSIONS: Although the presence of an unmutated IgV(H) gene is strongly associated with the expression of ZAP-70, ZAP-70 is a stronger predictor of the need for treatment in B-cell CLL.
Copyright 2026 Massachusetts Medical Society
PMID: 15329427
____________
Blood Journal Article
Blood. 2026 Dec 15;100(13):4609-14. Epub 2026 Aug 08.
Expression of ZAP-70 is associated with increased B-cell receptor signaling in chronic lymphocytic leukemia.
Chen L, Widhopf G, Huynh L, Rassenti L, Rai KR, Weiss A, Kipps TJ.
Division of Hematology/Oncology, Department of Medicine, University of California, San Diego for the CLL Research Consortium, San Diego, CA.
We examined isolated leukemia B cells of patients with chronic lymphocytic leukemia (CLL) for expression of zeta-associated protein 70 (ZAP-70). CLL B cells that have nonmutated immunoglobulin variable region genes (V genes) expressed levels of ZAP-70 protein that were comparable to those expressed by normal blood T cells. In contrast, CLL B cells that had mutated immunoglobulin variable V genes, or that had low-level expression of CD38, generally did not express detectable amounts of ZAP-70 protein. Leukemia cells from identical twins with CLL were found discordant for expression of ZAP-70, suggesting that B-cell expression of ZAP-70 is not genetically predetermined. Ligation of the B-cell receptor (BCR) complex on CLL cells that expressed ZAP-70 induced significantly greater tyrosine phosphorylation of cytosolic proteins, including p72(Syk), than did similar stimulation of CLL cells that did not express ZAP-70. Also, exceptional cases of CLL cells that expressed mutated immunoglobulin V genes and ZAP-70 also experienced higher levels tyrosine phosphorylation of such cytosolic proteins following BCR ligation. Following BCR ligation, ZAP-70 underwent tyrosine phosphorylation and became associated with surface immunoglobulin and CD79b, arguing for the involvement of ZAP-70 in BCR signaling. These data indicate that expression of ZAP-70 is associated with enhanced signal transduction via the BCR complex, which may contribute to the more aggressive clinical course associated with CLL cells that express nonmutated immunoglobulin receptors.
PMID: 12393534
____________
Until very recently, the conventional wisdom was that each of us was uniquely different, even in the exact type of our CLL. Let us get this point straight, while the word "clone" implies that each of the CLL cells in a given patient are all the same, it was thought that no two CLL patients would have the exact same shaped immunoglobulin (Ig) tips. In fact, the analogy was made to fingerprints: it is statistically next to impossible for two individuals to have the same fingerprints, and no two CLL patients could have the exact same Ig. This is one of the reasons why idiotype peptide vaccines are considered such an expensive proposition as each patient would need custom made vaccine to work with the specific Ig that his or her CLL cells have, and no other patient would match this exact shape of Ig.
Well, this is one concept that has been proven wrong in recent months. Below are four abstracts that make the same point, but the first one below by Ghiotto, Rai, Chiorazzi et. al., really caught my attention. It is a beautiful piece of detective work. I am really impressed by the quality of research coming out of North Shore Hospital in recent months (part of Long Island Jewish Hospital system, I believe). In case you are interested in reading the full text of this terrific article, please write to us. What I would give to be able to get these researchers answer a few questions for us and explain some of the more difficult concepts: connect the logic dots as it were.
Here are the nuts-and-bolts of the article. The authors examined the CLL cells from 25 different patients, all of whom expressed a subset of immunoglobulin called IgG (don't worry about exactly how IgM and IgG differ, for now. The differences are important, but the subject of another article). The chances that any two of these 25 CLL cases would just happen to have, by sheer coincidence, the exact same shaped pincers on the tips of the immunoglobulins expressed by the CLL cells is roughly one in 3.5 million. Imagine the surprise of the researchers when not two but five out of this set of 25 had absolutely identical shaped Ig pincers!! It is as if the detective in the murder story had five of the suspects matching exactly the fingerprints on the murder weapon. This is a major finding. Since CLL cells that have come in contact with an antigen express the pincer tips at the ends of their Ig to match that particular antigen, the unavoidable conclusion is that each of these five patients have CLL that reflects interaction with a common antigen!! Think what this means: for these five patients, there is a common thread, a common focal point at the heart of their CLL.
What could this common antigen be? It could be a self-antigen, something that is common to all five people, a piece of protein that exists as natural part of their bodies. "Normal" people can handle this self-antigen just fine, but in the case of people with some predisposition to cancer, the same self-antigen could spell CLL. Or it could be an environmental toxin that each of the five patients came into contact with, at some defining moment in their lives. Or it could be a protein fragment from a common virus or bacteria, something that is ubiquitous in the general population. The detective story unfolds: these distinct groups of CLL patients with this common theme to their CLL are not from one geographic location and probably not linked by trade or type of work. It is looking more and more as if the common antigen may be a viral or bacterial pathogen. Something that is probably common and present in most of humanity. We already know that once we are infected, viruses such as the Herpes Simplex virus, EBV, and hepatitis virus etc, lie dormant in us for the rest of our lifetimes. Many cancers have been shown to be caused by viral or bacterial pathogens, including some types of non-Hodgkin's lymphoma. Mononucleosis, which is characterized by uncontrolled proliferation of B-cells, is caused by Epstein-Barr virus (EBV), transmitted from person to person through saliva. The common name for it is the "kissing disease" for obvious reasons, and there is a reasonable level of research pointing to links between mononucleosis and the development of some kinds of B-cell lymphoma. The vast majority of mankind has learned how to deal with these antigens, except the unfortunate few that were susceptible, and in these folks this common antigen precipitated their CLL.
Why is this important? Think about it, while we cannot completely eradicate some viruses such as EBV and Herpes Simplex, in recent years we have learned how to control them, drive them into dormancy by anti-viral medications such as famcyclovir (Famvir). I am sure you have all seen recent advertisements for controlling genital or oral herpes outbreaks. I am using the Herpes Simplex and EBV viruses as an example only, the common antigen we are talking about in these 5 patients is entirely likely to be something different. But once we know what it is, we can attack the CLL from that perspective. Lock up the antigen, block it, drive it out, eradicate it, whatever. As long as it is not around to be a constant goad to the CLL cells, activating them and making them go into a state of proliferation, it will help in controlling the disease. CLL is caused by a small mismatch in the rate at which new cancer cells are created versus the rate at which they are killed. If we can slow down or stop the proliferation aspect of the equation, we will gain control over the situation, not just for these five patients but for all other patients who happen to fall into this specific group. The interesting news is that there may be many other such "clumps" of CLL patients, each clump corresponding to a particular pathogen. The same logic would work for all these people - it would be a matter of identifying the specific pathogen responsible for each clump of CLL patients.
Here is the question I would like to have answered by the expert researchers. In the absence of knowing the specific nature of the antigen that is implicated in these clumps of CLL patients, can we devise therapy strategies that use a broad spectrum approach, someway of neutralizing the antigen by a carpet bombing approach? Since the new research is suggesting the antigens in question are common and ubiquitous in the general population, it stands to reason that the general healthy public has found ways of dealing with them, otherwise we would have whole populations coming down with CLL. Can we then use the antigenic wisdom of the healthy population to protect the few of us who don't quite know how to do it for ourselves?
Immunoglobulins within our blood are a reflection of the lessons learned by our bodies as they faced down different antigens. Immunoglobulins collected from the blood of thousands of donors reflects the combined wisdom of all those donors. Between the lot of them, chances are good that they have battled most of the common antigens and won. Intravenous immunoglobulin (IVIg) infusions have been around for a long time, and have been used as broad spectrum protection against infections in immune compromised patients. In fact, CLL is one of the conditions listed for which IVIG is an approved therapy. Now I am asking the question if IVIG infusions have a second, and even more important role to play in a subset of patients by neutralizing the specific and common antigen that might be the driver for the CLL proliferation. IVIg is commercially available, and the recent introduction of better purification processes in collecting the immunoglobulins from pooled blood have made the products better, with less chance of adverse effects and allergic reactions. Surely this concept deserves evaluation? Some of you have sent me your medical records, and I thank you for the trust you show in doing so. I will never breach your confidentiality. The point is this: having access to many different scenarios of how the CLL plays out gives me a better perspective. Here then, is what I have observed: in at least a few anecdotal cases, a series of regular IVIg infusions (generally once a month) have led to a gradual and steady decrease in tumor load, a remission that has not needed any chemotherapy or other drugs, and therefore free of any side effects or toxicity. You can understand my level of interest in this concept!
J Clin Invest. 2026 Apr;113(7):1008-16.
Remarkably similar antigen receptors among a subset of patients with chronic lymphocytic leukemia.
Ghiotto F, Fais F, Valetto A, Albesiano E, Hashimoto S, Dono M, Ikematsu H, Allen SL, Kolitz J, Rai KR, Nardini M, Tramontano A, Ferrarini M, Chiorazzi N.
Department of Medicine, North Shore University Hospital and New York University School of Medicine, Manhasset, New York, USA.
Studies of B cell antigen receptors (BCRs) expressed by leukemic lymphocytes from patients with B cell chronic lymphocytic leukemia (B-CLL) suggest that B lymphocytes with some level of BCR structural restriction become transformed. While analyzing rearranged V(H)DJ(H) and V(L)J(L) genes of 25 non-IgM-producing B-CLL cases, we found five IgG(+) cases that display strikingly similar BCRs (use of the same H- and L-chain V gene segments with unique, shared heavy chain third complementarity-determining region [HCDR3] and light chain third complementarity-determining region [LCDR3] motifs). These H- and L-chain characteristics were not identified in other B-CLL cases or in normal B lymphocytes whose sequences are available in the public databases. Three-dimensional modeling studies suggest that these BCRs could bind the same antigenic epitope. The structural features of the B-CLL BCRs resemble those of mAb's reactive with carbohydrate determinants of bacterial capsules or viral coats and with certain autoantigens. These findings suggest that the B lymphocytes that gave rise to these IgG(+) B-CLL cells were selected for this unique BCR structure. This selection could have occurred because the precursors of the B-CLL cells were chosen for their antigen-binding capabilities by antigen(s) of restricted nature and structure, or because the precursors derived from a B cell subpopulation with limited BCR heterogeneity, or both.
PMID: 15057307
____________
J Exp Med. 2026 Aug 16;200(4):519-25.
Multiple distinct sets of stereotyped antigen receptors indicate a role for antigen in promoting chronic lymphocytic leukemia.
Messmer BT, Albesiano E, Efremov DG, Ghiotto F, Allen SL, Kolitz J, Foa R, Damle RN, Fais F, Messmer D, Rai KR, Ferrarini M, Chiorazzi N.
North Shore-LIJ Research Institute, 350 Community Dr., Manhasset, NY
Previous studies suggest that the diversity of the expressed variable (V) region repertoire of the immunoglobulin (Ig)H chain of B-CLL cells is restricted. Although limited examples of marked constraint in the primary structure of the H and L chain V regions exist, the possibility that this level of restriction is a general principle in this disease has not been accepted. This report describes five sets of patients, mostly with unmutated or minimally mutated IgV genes, with strikingly similar B cell antigen receptors (BCRs) arising from the use of common H and L chain V region gene segments that share CDR3 structural features such as length, amino acid composition, and unique amino acid residues at recombination junctions. Thus, a much more striking degree of structural restriction of the entire BCR and a much higher frequency of receptor sharing exists among patients than appreciated previously. The data imply that either a significant fraction of B-CLL cells was selected by a limited set of antigenic epitopes at some point in their development and/or that they derive from a distinct B cell subpopulation with limited Ig V region diversity. These shared, stereotyped Ig molecules may be valuable probes for antigen identification and important targets for cross-reactive idiotypic therapy.
PMID: 15314077
____________
Blood. 2026 Jun 24
Chronic Lymphocytic Leukemia B Cells of Over One Percent of Patients Express Virtually Identical Immunoglobulins.
Widhopf II GF, Rassenti LZ, Toy TL, Gribben JG, Wierda WG, Kipps TJ.
Department of Medicine, Division of Hematology/Oncology, University of California, San Diego School of Medicine, La Jolla, CA, USA; The CLL Research Consortium, USA.
We examined the immunoglobulin (Ig) heavy chain variable region genes (V(H) genes) used by leukemia cells of 1,220 unrelated patients with chronic lymphocytic leukemia (CLL). We found 1,188 (97%) expressed Ig encoded by a single IgV(H) subgroup, the most common of which was V(H)3 (571 or 48.1%), followed by V(H)1 (319 or 26.8%) and V(H)4 (241 or 20.2%). Using allele-specific primers, we found 13.8% of all samples (n = 164) used one major V(H)1-69 allele, designated 51p1, 163 of which were not somatically mutated. For these cases there was marked restriction in the structure of the Ig third complementarity determining regions (CDR3), which were encoded by a small number of unmutated D and J(H) gene segments. Strikingly, 15 of the 163 cases had virtually identical CDR3 encoded by the second reading frame of D3-16 and J(H)3. Further analysis revealed that each of these 15 samples used the same unmutated Ig kappa light-chain gene, namely A27. These data reveal that ~1.3% (15/1,220) of all patients had leukemia cells that expressed virtually identical Ig. This finding provides compelling evidence that the Ig expressed by CLL B cells are highly selected and not representative of the Ig expressed by naive B cells.
PMID: 15217828
____________
Blood. 2026 Jun 15;101(12):4952-7. Epub 2026 Feb 13.
Tobin G, Thunberg U, Johnson A, Eriksson I, Soderberg O, Karlsson K, Merup M, Juliusson G, Vilpo J, Enblad G, Sundstrom C, Roos G, Rosenquist R.
Department of Genetics and Pathology, Uppsala University, Sweden.
The immunoglobulin variable heavy chain (IgVH) gene mutation status is an important prognostic factor in chronic lymphocytic leukemia (CLL), since cases with mutated VH genes show significantly longer survival than unmutated cases. Recently, we reported a preferential use of the VH3-21 gene in mutated CLL and showed that mutated VH3-21 cases had an inferior overall survival compared with other mutated CLL. In order to further characterize this subset, we performed VH gene analysis in 265 CLL cases and identified 31 VH3-21 cases (11.7%); 21 cases had mutated and 10 cases unmutated VH genes. Regardless of VH gene mutation status, a poor overall survival was found in the VH3-21 cases with a median survival of 83 months. These survival data confirm that VH3-21 cases do not fit into the general prognostic grouping of mutated and unmutated CLL. A large fraction of VH3-21 cases also demonstrated unique features with shorter lengths of the third complementarity determining region (CDR3) and CDR3s with highly homologous amino acid sequences. Furthermore, the VH3-21 cases showed a striking dominance of lambda light chain expression, and analysis of the Iglambda gene rearrangements revealed highly restricted use of the Vlambda2-14/Jlambda3 genes in the majority of cases. Taken together, our new findings strengthen the suggestion that VH3-21-using cases comprise a new CLL entity, irrespective of VH gene mutation status, and implicate that a common antigen epitope, perhaps of pathogenic significance, is recognized by the highly homologous VH3-21/Vlambda2-14 Ig molecules expressed in individual tumors.
PMID: 12586612
____________
 Enter Keywords: |
———
Disclaimer: The content of this website is intended for information only and is NOT meant to be medical advice. Please be sure to consult and follow the advice of your doctors on all medical matters.
Copyright Notice:
Copyright © 2026-2007 CLL Topics, Inc. All Rights Reserved.
All materials contained on this site are protected by United States copyright law and may not be reproduced, distributed, transmitted, displayed, published or broadcast without the prior written permission of CLL Topics, Inc. You may not alter or remove any trademark, copyright or other notice from copies of the content.
However, you may download and print material from CLLTopics.org exclusively for your personal, noncommercial use.
———
