 |
||||||||||
Date: November 15, 2026
by Chaya Venkat
Related Article: Killer T-Cells, Up Close and Personal

Once every few years, there is an “Aha!” moment in science when a new breakthrough happens and for a while there is a palpable sense of excitement and urgency. Sometimes the “Aha!” turns out to be no more than a fad that soon peters out. But sometimes the new perspective becomes the starting point for a startlingly broad new perspective that translates into major achievements. There has been one such development in immunology in the past few years, one that has major therapy implications for cancer patients like us. In the years to come, you will be hearing a lot more about Regulatory T-cells (Tregs for short), and the role they play in treating CLL. This is an important subject, one that explains some of the risks and rewards associated many of the drugs in use right now.
CLL is a cancer of the B-cell compartment of the immune system. (There is also T-cell CLL, which has much smaller incidence and is a beast of a different color that we do not discuss much on this website). But B-cells and T-cells have a very cozy relationship. Not only does the CLL itself corrupt the proper functioning of all other aspects of the immune system, many of the drugs used to treat CLL are devastating to T-cell populations. At the top of the list of T-cell killing drugs are Campath and fludarabine.
In a nutshell, T-cells are one of the most important components of our immune system. T-cells are smart, targeted and very sophisticated shock troops, and their proper function is crucial for maintaining health. A major part of their work is seeking out and destroying cells that have been infected by viruses, as well as nipping in the bud any small population of cancer cells, before the problem gets out of hand. People with defective T-cell function (such as AIDS patients, or CLL patients undergoing immune suppressive therapy) are much more prone to viral infections (pneumonia and the like) and other pathogens as well as secondary cancers (skin cancer is at the top of the list).
It has been known for some time that not all T-cells are the same. The complexity of all the varieties of T-cells is beyond the scope of this article. For our present purpose, it is enough to know that there are killer T-cells, potent and dangerous, able to identify and kill invading viruses and other pathogens, as well as home grown cancer cells. You can learn more about them by going to an earlier article on the subject (Killer T-cells and CTL Therapy). They are so potent that they can do major damage to our own bodies, if they were not appropriately controlled. This regulatory function is done by another subset of T-cells, called “regulatory T-cells” or Tregs.
Many of us in the United States have had recent occasion to ponder the roles of the executive and legislative branches of our government, as we made our voting decisions couple of weeks ago. A good balance of power, appropriate checks and balances, are part of our constitution. The analogy between good governance and healthy functioning of the immune system is actually a pretty good one, if not quite politically correct. A vibrant executive branch (immune system), willing and able to defend us against foreign invaders and terrorists (viruses, pathogens), as well as the home-grown variety of malcontents (cancer cells), is very important for security of the nation (good health). However, there is fine line between vigilance and over-the-top aggressive paranoia. Quality of life will suffer (autoimmune disease) if innocent and perfectly legitimate citizens are “profiled” and targeted by an overzealous security apparatus, in too much of hurry to take action and unwilling to take the time to tell the difference between friend and foe. Checks and balances are needed to keep the executive branch (killer T-cells) under control and protect the constitutional rights of law-abiding citizens from shoot-first-and-ask-questions-later security forces. But at the same time, you can understand that too much regulatory oversight (by Tregs) may cause unmitigated grid-lock, hobbling our government (immune system) so that it cannot act even when the enemy visibly breaches our defenses (viral infections, cancer). You get the picture. Good health and good governance requires a well-balanced system where neither gung-ho offensive forces nor stifling regulatory forces are free to do exactly what they want.
I got into an interesting conversation with a soft-spoken older gentleman in the course of my recent travels in India. I was curious to know what the rest of the world made of our upcoming US mid-term elections and the issues involved. My travel companion had an interesting observation: he had never been outside of India, and to his inexperienced eye it was hard telling apart the various personalities. To him, one pasty-faced and over-weight “mature” white guy looked pretty much like another, it was hard telling apart the faces of “warrior” Rumsfeld, “anti-terrorist” Cheney and “crusading congressman” Murtha on the television. Indian names are often very descriptive (often including the name of the village, name of the father, clan, etc., in the full nine-yard long version, which is why my husband goes by his initials, “PC”), but American names don’t give you much of a handle, according to my traveling acquaintance. He was pretty sure Mr. Baker did not know too much about making bread. How can you figure out anything, he said, if they all look alike and even their names don’t say who they are, what caste, where they come from?
A similar problem faced early research into the different sub-sets of T-cells. As early as 1971, Gershon and Kondo identified so-called "suppressor” T-cells. Even in the early 1990s, T-cells with regulatory function were reported in patients with cancer. However, this early research gave conflicting and confusing results. It was not until mid to late-1990s, until the identification of the CD4+ CD25+ phenotype that research into Tregs started yielding valuable insights. Assessment of the most specific marker — namely, FOXP3 — did not happen until very recently, and this is a major defect in many of the early studies. The most recent research articles define Tregs as CD4+ CD25high FOXP3+, a minimum requirement for accurate naming of this important group of T cells. No doubt the phenotype will be even further refined as we go forward, making the name even longer. For our purposes, it is not all that important that we know the full functional meaning of these various markers. Think of the phenotype description as first, second and family name, necessary for accurate identification. For the sake of simplicity, many researchers call them just “Tregs” and the detailed phenotype is assumed. Now that we have a better handle on identifying them, cutting-edge research into how they influence the immune system function has become tremendously exciting.
As we said above, one of the functions of good T-cell surveillance is killing cancer cells and not letting the disease gain a toe-hold within our bodies. Yet, people do get cancer, so something is not working right in these people. It was thought that cancer cells are so similar to normal cells that they can hide from the killing power of T-cells, melt away into the general crowd and not be seen sticking out like a sore thumb. Now that conventional wisdom is giving way to a new perspective. Cancer cells are indeed (easily) recognizable by the immune system as dangerous. They can be killed by killer T-cells — if only the killer T-cells are permitted to do so. The question is what is stopping the killer T-cells from getting rid of the cancer cells? I am sure you have guessed the answer by now. Tregs are putting the brakes on this important process. Vastly increased numbers of Tregs are observed in cancer patients (in both solid cancers and blood cancers). There are leads suggesting that the cancer cells actively help the creation of increased T-reg populations, as a mechanism of protecting themselves from the just wrath of killer T-cells. The abstract below brings it into perspective. You can read the full article by clicking on the link provided.
Blood Journal Article
1: Blood. 2026 Sep 15;106(6):2018-25. Epub 2026 May 24.
Reduced frequencies and suppressive function of CD4+CD25hi regulatory T cells in patients with chronic lymphocytic leukemia after therapy with fludarabine.
Beyer M, Kochanek M, Darabi K, Popov A, Jensen M, Endl E, Knolle PA, Thomas RK, von Bergwelt-Baildon M, Debey S, Hallek M, Schultze JL.
Molecular Tumor Biology and Tumor Immunology Clinic I for Internal Medicine, University of Cologne, Joseph-Stelzmann Str 9/Haus 16, 50931 Cologne, Germany.
Globally suppressed T-cell function has been described in many patients with cancer to be a major hurdle for the development of clinically efficient cancer immunotherapy. Inhibition of antitumor immune responses has been mainly linked to inhibitory factors present in cancer patients. More recently, increased frequencies of CD4+CD25hi regulatory T cells (Treg cells) have been described as an additional mechanism reducing immunity. We assessed 73 patients with B-cell chronic lymphocytic leukemia (CLL) and 42 healthy controls and demonstrated significantly increased frequencies of cytotoxic T lymphocyte-associated protein 4 (CTLA4+)-, Forkhead box P3 (FOXP3+)-, glucocorticoid-induced tumor necrosis factor receptor-related protein (GITR+)-, CD62L+-, transforming growth factor beta1 (TGF-beta1+)-, interleukin 10 (IL-10+)-Treg cells in patients with CLL, with highest frequencies in untreated or progressing patients presenting with extended disease. Most surprisingly, in the majority of patients with CLL treated with fludarabine-containing therapy regimens the inhibitory function of Treg cells was decreased or even abrogated. In addition, frequencies of Treg cells were significantly decreased after therapy with fludarabine. In light of similar findings for cyclophosphamide the combination of fludarabine and cyclophosphamide might be further exploited in strategies reducing immunosuppression prior to cancer immunotherapy.
PMID: 15914560 [PubMed - indexed for MEDLINE]
____________
Here are some highlights from this important paper:
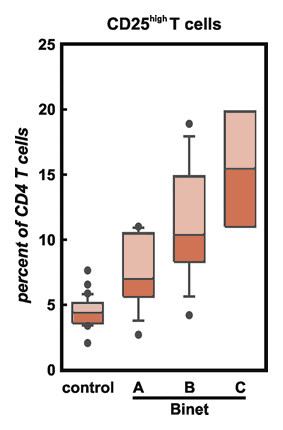
Tregs as a Percentage of T-cells
But there is another side to the coin. Another consequence of reduced T-reg surveillance is disproportionate and over-the-top aggressiveness of killer T-cells. You know the saying, mice will play when the cat is away. Without proper checks and balances, patients with an insufficient number of Tregs are prone to autoimmune disease, in which perfectly good cells of the body are destroyed. Autoimmune hemolytic anemia (AIHA) and thrombocytopenia (ITP) are two familiar examples. A strong case has been made for increased risk of AIHA in patients who have undergone fludarabine therapy as long ago as 1995. Now we have a better handle on why this is the case.
Br J Haematol. 1995 Oct;91(2):341-4.
Fludarabine-related autoimmune haemolytic anaemia in patients with chronic lymphocytic leukaemia.
Myint H, Copplestone JA, Orchard J, Craig V, Curtis D, Prentice AG, Hamon MD, Oscier DG, Hamblin TJ.
Department of Haematology, Royal Bournemouth Hospital, United Kingdom.
We have treated 52 patients with chronic lymphocytic leukaemia (CLL) with fludarabine; 12 developed severe autoimmune haemolysis. Only three had a previous history of haemolytic anaemia. Six out of eight patients retreated with fludarabine after control of their haemolysis developed an exacerbation of the haemolytic anaemia. The cause of autoimmune phenomena in CLL is not known, but our findings reinforce the view that they are caused by a disturbance in immunoregulatory T cells. Fludarabine is a known suppressor of T-cell function.
PMID: 8547072
___________
Along with fludarabine, the other big T-cell killer is Campath (Campath Therapy). Is there a similar risk of autoimmune disease after Campath-based therapy? Unlike fludarabine, Campath and its sister monoclonal antibody, Rituxan, have been around only for a few years and downstream adverse reactions like the onset of autoimmune disease take a while to surface, if they exist. I have recently heard from three of our members in quick succession, patients who have had Campath therapy and then were diagnosed with ITP (autoimmune thrombocytopenia, where platelets are destroyed by the immune system). It seemed a bit of a coincidence to me and so I looked into it. I found no research papers linking Campath to autoimmune disease, in CLL patients. However, and this is very interesting, there have been documented cases of ITP in patients with multiple sclerosis after they have undergone Campath therapy. So much so that the risk of ITP is now a mandated warning on the Campath label — but only for multiple sclerosis patients. If I were to read these tea leaves, it may be that people with prior risk factors for autoimmune disease (multiple sclerosis is an autoimmune disease in which nerve cells are destroyed by the immune system) are well advised to think twice about this issue prior to undertaking Campath therapy. If you are a CLL patient and you already have autoimmune complications such as AIHA, perhaps Campath poses more of a risk to you.
Only time will tell if there is validity to this concern. There is little doubt that Campath destroys T-cells of all persuasions immediately after its administration. Patients have to be on protective antibioitcs, anti-virals and anti-fungals during this period of vulnerability. Even 12 months after completion of Campath therapy, various T-cell counts are at less than 25% of their pre-treatment levels. Now we are beginning to see further details to this T-cell recovery. In patients who are prone to autoimmune disease, as in MS patients, there may be a biased distribution of the T-cells that do recover after Campath therapy, a bias that works against T-reg recovery relative to killer T-cells and this may precipitate life-threatening ITP.
FDA Campath warning - ITP risk in MS patients
This FDA warning is now reflected in the “Black-box" warning on the Campath label. Black-box warnings are the highest form of FDA drug safety alert, and, because of their importance, usually appear at the top of a drug's package insert, or label. Typically the black-box warning brings attention to serious side effects associated with the drug and provides guidance about how to minimize the risks of those side effects. Right now the warning pertains only to use of Campath for multiple sclerosis patients. No such warning exists for CLL patients. Yet, the only reason why I even looked up this whole business is because three separate CLL patients wrote to me about their experience of ITP soon after Campath therapy. Until we have more definitive long-term surveillance data on this drug, this will have to remain in the realm of an interesting but unproven “coincidence”.
OK, we can see how in individuals prone to autoimmune disease (as in late stage CLL patients, or those who are prone to this problem from inception), getting rid of Tregs is not such a smart thing to do. But how about people with early stage CLL, or those that have shown no tendencies toward autoimmune disease? For these guys, would it help to swing the balance of power a little bit more towards the killer T-cells, take away some of the over-zealous regulatory powers of Tregs? Would that give us an immune system better able to seek and destroy cancer cells (CLL cells, incipient skin cancer cells, etc.) as well as better protection from pesky viral infections and other pathogens? The abstract below authored by experts from Mayo Clinic, explores this very issue.
Future Oncol. 2026 Jun;2(3):379-89.
Strong-arming immune regulation: suppressing regulatory T-cell function to treat cancers.
Knutson KL.
Department of Immunology, Mayo Clinic College of Medicine, 342C Guggenheim, 200 First St. SW, Mayo Clinic, Rochester, MN.
In recent years there has been an accelerated understanding of immune regulatory mechanisms. Much of this immune regulation is linked to a collection of specialized regulatory cells of the T-cell lineage (Tregs). This collection consists of Tregs that are either thymically derived or peripherally induced. Tregs are important for controlling potentially autoreactive immune effectors and immune responses to foreign organisms and molecules. Their importance in maintaining immune homeostasis and the overall health of an organism cannot be overstated. However, there is a dark side, and Tregs may also be involved in the pathogenesis of malignancies. Evidence shows that tumors induce or recruit Tregs to block antitumor effectors. Thus, there are efforts underway to identify approaches that specifically inhibit the function of intratumoral Tregs, which could lead to increased immunity to tumors without off-target immune-related pathologies (i.e., autoimmune disease). In this review, the biology of Tregs is discussed along with their involvement in malignancies and emerging strategies to block their function.
PMID: 16787118 [PubMed - indexed for MEDLINE]
____________
A very recent and detailed article in the prestigious journal “Blood” describes the importance of controlling Tregs in the treatment of cancer. The abstract is given below, and you can read the full article for free by clicking on the link provided.
Blood Journal Article
Blood. 2026 Aug 1;108(3):804-11.
Regulatory T cells in cancer.
Beyer M, Schultze JL.
Molecular Tumor Biology and Tumor Immunology Clinic I for Internal Medicine, University of Cologne, Joseph-Stelzmann Str 9/Haus 16, 50931 Cologne, Germany.
Increasing evidence supports the existence of elevated numbers of regulatory T cells (T(reg) cells) in solid tumors and hematologic malignancies. Whereas the biology of CD4(+)CD25(+)FOXP3(+) T(reg) cells in murine models seems to be rather straightforward, studies in human diseases are more difficult to interpret due to expression of CD25 on activated effector T cells as well as T(reg) cells. More importantly, early studies in human tumors were mainly focused on CD4(+)CD25(+) T(reg) cells lacking interrogation of more specific markers such as FOXP3 expression. Although the increase of T(reg) cells seems to be a characteristic feature in most tumors, little is known about the molecular and cellular mechanisms responsible for the increase and maintenance of elevated levels of T(reg) cells in cancer. We will discuss earlier data in the context of recent findings in T(reg)-cell biology with a particular emphasis on CD4(+)CD25(high)FOXP3(+) T(reg) cells in human malignancies.
PMID: 16861339
____________
I have taken the liberty of reproducing a very interesting chart from this paper. It describes the role of various drugs and chemotherapy agents in terms of their effect on Tregs. As expected, fludarabine is shown to deplete Tregs. But not as well known is the role of cyclophosphamide in doing the same thing.
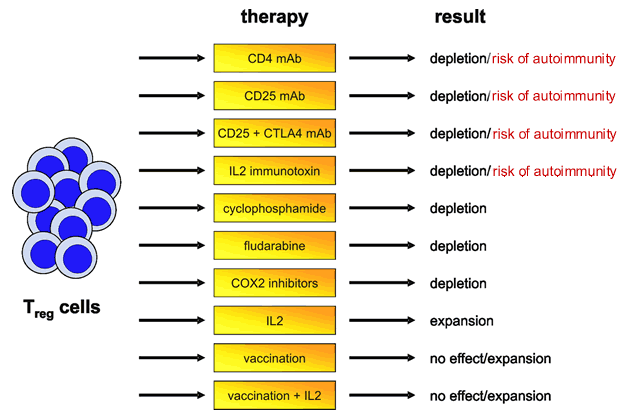
Effect of Therapy on Tregs
It has long been recognized that cyclophosphamide stimulates the immune system. Early data indicated that cyclophosphamide preferentially destroys Tregs, permitting killer T-cells to destroy lymphomas in mice. In a rat colon cancer model, administration of cyclophosphamide depleted Tregs and gave longer remissions. Combining cyclophosphamide with immunotherapy drugs even cured the mice, whereas each drug applied alone had no curative effect. Low-dose cyclophosphamide (the so called “metronomic administration”, where the drug is given in very small amounts long term, on a daily basis) not only decreases numbers of Tregs but also decreased their ability to function, and proliferate.
Cyclophosphamide (“Cytoxan”) is an alkylating agent, similar in that sense to chlorambucil (“Leukeran”). In recent years, cyclophosphamide has seen an increased use in combinations such as FCR and PCR. Its ability to suppress Tregs may add to the effectiveness of the combinations.
Blood Journal Article
Blood. 2026 Apr 1;105(7):2862-8. Epub 2026 Dec 9.
Inhibition of CD4(+)25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide.
Lutsiak ME, Semnani RT, De Pascalis R, Kashmiri SV, Schlom J, Sabzevari H.
Laboratory of Tumor Immunology and Biology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bldg 10, Rm 8B09, Bethesda, MD.
Regulatory T cells (T(REGs)) control the key aspects of tolerance and play a role in the lack of antitumor immune responses. Cyclophosphamide (CY) is a chemotherapeutic agent with a dose-dependent, bimodal effect on the immune system. Although a previous study demonstrated that CY reduces the number of T(REGs), the mechanism involved in this process has yet to be defined. In this report, it is established that low-dose CY not only decreases cell number but leads to decreased functionality of T(REGs). CY treatment enhances apoptosis and decreases homeostatic proliferation of these cells. Expression of GITR and FoxP3, which are involved in the suppressive activity of T(REGs), is down-regulated after CY administration, though the level of expression varies depending on the time studied. This is the first report demonstrating that CY, in addition to decreasing cell number, inhibits the suppressive capability of T(REGs). The relevance of the loss of suppressor functionality and the changes in gene expression are further discussed.
PMID: 15591121
____________
Because Tregs are an important cellular mechanism for controlling killer T-cells from attacking innocent bystander cells, heavy-handed depletion of these cells might be too crude an approach to be used without leading to significant collateral damage and autoimmune disease. Equally important, mere control of Tregs may be insufficient to “cure” well established cancers that have found multiple pathways of avoiding attack by killer T-cells. Although there may be many factors that contribute to the induction of AIHA, recent research indicates that loss of T-reg function could play an essential role in the onset and/or maintenance of this autoimmune disease. Current therapies for AIHA include administration of immunosuppressive drugs, as well as transfusions or splenectomy, therapies that typically control, rather than cure, the disease. Better (finer) control over the function of Tregs may represent a more effective strategy for treatment of AIHA.
Another area where killer T-cell function has to be carefully matched by T-reg control is in the context of stem cell transplant. Mini-allo transplants (Only Real Cure, Graft versus Host Disease, GVHD Developments) depend on the graft-versus-leukemia effect to eradicate the last remaining traces of cancer cells, where killer T-cells of the newly grafted immune system finish the job. However, if these newly grafted killers are not adequately controlled, the result is mayhem and very debilitating graft-versus-host disease. Successful transplants need effective GVL, but with GVHD kept under control. Researchers are beginning to hope that we can have our cake and eat it too — have potent GVL to cure the cancer without precipitating life-threatening GVHD. Indeed, the future looks bright for mini-allo transplants in CLL.
Notice that the chart above lists Cox-2 inhibitors also as good suppressors of Tregs. Cox-2 inhibitors have been in the news a great deal in recent months (Vioxx, Celebrex). Much of their attractivness as risk-free pain killers (less damage to the GI tract, less problems with stomach bleeding) has been destroyed by new information on their potential for increased risk of heart disease. Leaving aside their GI friendly (?) but not so heart friendly issues for a moment, there has been persistent interest in these drugs as potential cancer therapies. Their newly defined ability to control Tregs, and consequent ability to give killer T-cells more free rein to kill cancer cells may explain their cancer fighting profile. Here is a very interesting study where a small molecule drug was combined with Celebrex, with a terrific improvement in response and survival of late stage lung cancer: Tarceva plus Celebrex to Treat Lung Cancer.
As background, non-small cell lung cancer is a very tough cancer. Those with advanced disease usually survive less than a year. Tarceva is a targeted drug that has improved chances for these patients. It is an orally taken pill designed to block tumor cell growth by blocking a protein called “Human epidermal growth factor receptor”. In this very interesting study, patients who were on Tarceva + Celebrex had threefold increased response rate, up from 10% to 33%. The longest duration was 93 weeks, which does not sound like much until you consider that people who got Tarceva alone had remissions that were around 30 weeks long.
I am beginning to hear of interest in combining targeted CLL drugs (like Rituxan, or HuMax-CD20) with T-reg inhibiting adjuvants like Celebrex. True, the long term and high dose use of Cox-2 inhibitors is associated with increased risk of heart disease. But that risk is significantly reduced if the duration of Celebrex use is limited to just a few weeks. In any case, one needs to weigh the real risks of CLL with the significantly much smaller risks of cardiac toxicity of a drug such as Celebrex. Would you consider taking Celebrex for a short time, as you go through anti-CD20 monoclonal therapy? Would the small increase in risk of cardiac toxicity more than balance the potential reward of improved CLL response? Good question, and one that I would urge you not to try to answer on your own without adult (read medical) supervision. But it is a concept that I would dearly like to see addressed in a properly conducted clinical trial. Would you participate in such a clinical trial (Rituxan or HuMax-CD20 + Celebrex), if you had a chance? Would you consider it a good thing if CLL Topics were to sponsor such a clinical trial with your hard earned donations? Let us know: this is not a far-fetched idea and we have already gotten a few highly regarded researchers interested in this concept.
I cannot end this article without a word of caution. Our understanding of T-reg function is growing rapidly but there are many areas where we have yet to solve the mysteries. For starters, much of the work reported even just a few years ago (prior to better definition of Tregs) is unreliable and possibly inaccurate. Bear that in mind if you go looking up Tregs on PubMed — take articles published earlier than 2026 with a large pinch of salt. New breakthroughs and better understanding of how Tregs function will lead to more customized approaches to cancer and autoimmune disease, allowing us to establish a healthier equilibrium between the two sides of the same coin.
Here are a couple of additional references that you might find interesting:
Eur J Immunol. 2026 Oct 19;36(11):2832-2836 [Epub ahead of print]
FOXP3(+) regulatory T cells: Current controversies and future perspectives.
Banham AH, Powrie FM, Suri-Payer E.
Nuffield Department of Clinical Laboratory Sciences, University of Oxford, John Radcliffe Hospital, Oxford, UK.
Regulatory T cells (Treg) provide protection from autoimmune disease, graft-versus-host disease, transplant rejection and overwhelming tissue destruction during infections. Conversely, high Treg numbers enable cancer cells to evade the host immune response. Thus, Treg are seen as an important tool to manipulate the immune response. However, as the immunological community is trying to move this knowledge from mice to humans, contradictory results regarding the number and function of Treg in various diseases are appearing. This problem arises because we cannot clearly define Treg populations on the basis of expression of CD25 and other cell surface markers in humans. This review addresses the utility of the FOXP3 forkhead transcription factor for the identification of Treg populations and summarizes recent data on the expression of FOXP3 in lymphomas. It is crucial to really understand Treg biology before attempting therapies, including (i) the injection of expanded Treg to cure autoimmune disease or prevent graft-versus-host disease or (ii) the depletion or inhibition of Treg in cancer therapy. For instance, new data arising from the study of haematological malignancies highlight the additional complexity of systems where malignant cell populations may also be direct Treg targets.
PMID: 17051620
____________
Cellular Imaging Research
Movie of Treg Activity
Regulatory T cells reversibly suppress cytotoxic T cell function independent of effector differentiation
Massachusetts General Hospital, Harvard University
PMID: 16860762 [PubMed - indexed for MEDLINE]
____________
 Enter Keywords: |
———
Disclaimer: The content of this website is intended for information only and is NOT meant to be medical advice. Please be sure to consult and follow the advice of your doctors on all medical matters.
Copyright Notice:
Copyright © 2026-2007 CLL Topics, Inc. All Rights Reserved.
All materials contained on this site are protected by United States copyright law and may not be reproduced, distributed, transmitted, displayed, published or broadcast without the prior written permission of CLL Topics, Inc. You may not alter or remove any trademark, copyright or other notice from copies of the content.
However, you may download and print material from CLLTopics.org exclusively for your personal, noncommercial use.
———
