 |
||||||||||
Date: December 15, 2026
by Chaya Venkat
Related Article: JCO Editorial by Byrd, et al.
There have been many recent articles about “Treanda”, the “new” chemotherapy drug for CLL. I suppose the company’s presentations at the recent ASH 2026 conference had a lot to do with it. For a fact, the stock price of the company has been going up, and there is a lot of buzz on the street. But is the hype justified? Are we going to remember this latest miracle of the month once we are past the Wall Street silly season? Is the FDA going to approve this drug for frontline therapy — and are generations of CLL patients going to be treated with it down the road? Will it improve our lot, extend our survival? Some of these questions need a crystal ball, and mine just broke the other day. But I will do my best to read the tea-leaves for you, get you a sense of the lay of the land without all the marketing buzz.
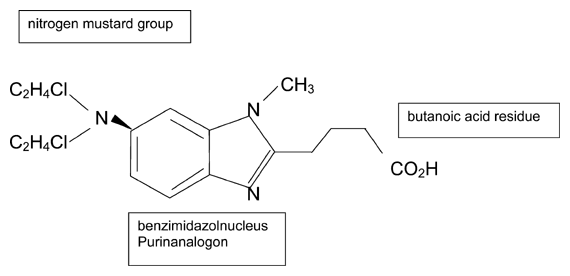
“Treanda” is the brand name of a drug that was invented long before the fall of the Berlin wall. Bendamustine hydrochloride was developed in the 1960s in the former East Germany but was never systematically studied in patients until the 1990s. The drug consists of a nitrogen mustard group that gives it properties of an alkylating agent (other such drugs you are familiar with are cyclophosphamide and chlorambucil) and a purine-like benzimidazol group that gives it properties like other purine analogs, such as fludarabine and pentostatin. In other words, it is not unreasonable to think of bendamustine as a one-two punch of both an alkylating agent and a purine analog, in one potent package. Below is the chemical structure, for those of you who share my passion for chemistry.
Chemical Structure of Bendamustine
Nature, 2026.
As we said, bendamustine has been around for a while, and it has seen its share of clinical trials – mostly in a variety of solid cancers, some blood cancers and mostly conducted in Europe. I suppose it was only a matter of time before they got around to testing this drug for CLL. You can find any number of recent quasi-technical / PR reports on the subject of bendamustine and CLL by doing a Google search (there will be far fewer if you do a similar search on PubMed). I will limit myself to a credible and detailed article published in the journal Haematologica in 2026 by the respected German CLL Study Group, as well as the two ASH 2026 abstracts published just a couple of weeks ago. I suspect much of the “buzz” is derived from the two ASH abstracts. First, let us walk through the Haematologica abstract since there is more meat here than in either of the ASH abstracts. A free link to the full-text article is provided below. Do let us know if you have trouble accessing it in your browser.
Haematologica. 2026 Oct;90(10):1300A.
Efficacy of bendamustine in patients with relapsed or refractory chronic lymphocytic leukemia: results of a phase I/II study of the German CLL Study Group.
Bergmann MA, Goebeler ME, Herold M, Emmerich B, Wilhelm M, Ruelfs C, Boening L, Hallek MJ;
German CLL Study Group. Medical Clinic III, University Hospital Grosshadern, Ludwig-Maximilians-University Munich.
BACKGROUND AND OBJECTIVES: Although bendamustine has been used for more than 30 years in the treatment of lymphoma, little is known about the optimal dosing schedule in relapsed or refractory B-cell chronic lymphocytic leukemia (CLL). Various dose and treatment schedules have been used empirically, and several phase II studies have shown impressive efficacy. To determine the maximal tolerated dose, dose-limiting toxicity and the optimal therapeutic dose of bendamustine for further phase III clinical trials the GCLLSG designed a phase I/II study for pre-treated CLL patients.
DESIGN AND METHODS: Sixteen patients (median age 67 years) with relapsed or refractory CLL were enrolled. All patients had been pre-treated with a median of three different regimens. Bendamustine was given at a starting dose of 100 mg/m2 on day 1 and 2, repeated every 3-4 weeks.
RESULTS: Major toxicities were leukocytopenia (CTC grade 3+4) in 8/16 and infections (CTC grade 3+4) in 7/16 patients. Six patients had dose-limiting toxicity which led to dose de-escalation from 100 to 70 mg/m2 in three patients. The maximum tolerated dose was 70 mg/m2. According to NCI-WG criteria, 9/16 patients (56%) responded to therapy, seven to doses <or=80 mg/m2. Two patients achieved complete remission. Five patients had a partial response and two patients stable disease. The median duration of response was 42.7 months. After a follow-up period of 53.2 months, five patients (31%) were still in remission. The median overall survival time for all patients was 45.6 months.
INTERPRETATION AND CONCLUSIONS: The study confirms the excellent efficacy of bendamustine in heavily pre-treated and treatment-refractory patients, even at reduced doses of 140 mg/m2 per course. In pre-treated CLL patients, impaired bone marrow function is likely to enhance the myelotoxic side effects of bendamustine. Based on these results, the recommended optimal therapeutic dose of bendamustine in refractory CLL is 70 mg/m2 on days 1 and 2 every 4 weeks.
PMID: 16219572
____________
One of the major reasons for interest in bendamustine is that it seems to have low cross-resistance with other alkylating agents (chlorambucil, cyclophosphamide) and with fludarabine. In other words, folks who have flunked fludarabine and/or cyclophosphamide or chlorambucil may still hope to get a response to this drug. That makes it an interesting drug for us since, it is always a good idea to have more options – especially for our refractory folks.
Doses of bendamustine of anywhere between 100 mg/m2 and 120 mg/m2 (or higher) given on days 1 and 2 of a 28-day cycle have been reported in treating NHL or a variety of solid cancer patients. But no one had looked specifically at the appropriate dosage for CLL patients until this carefully conducted study.
Sixteen patients were recruited. All of them had received at least one prior therapy with chlorambucil or fludarabine and had relapsed within 6 months of prior therapy. Most of the patients had much more than that under their belt, in fact all of them had three or more regimens of treatment. At study entry ten patients were classified as having Binet stage C disease and six patients as having stage B. These were patients who have been through the chemo wars and many of them showed the wear and tear associated with that experience. Several of them had co-morbidities that you would expect, such as hypertension, anemia, chronic sinusitis, etc.
The starting dose of bendamustine for these patients was 100 mg/m2 given on days 1 and 2 , and repeated every 3-4 weeks. (If you are not familiar with the mg/m2 notation, it is time you read and understood what it means. Body Surface Area or BSA determines the amount of drug tailored for your body size). The first cohort of three patients was treated with the starting dose of 100 mg/m2 bendamustine intravenously over 30 minutes on days 1 and 2. The idea was that if these guys handled it OK, the next cohort would get a more “manly” dose, and so on, until they hit the wall in terms of ‘acceptable’ toxicity. This is how researchers find “maximum tolerated dose” and “dose limiting toxicity”.
As it turned out, even the first level of 100mg/m2 on days 1-2 proved too much for the first cohort. Suffice to say, there was considerable dose-limiting toxicity and the decision was made to reduce the dose to 90 mg/m2 on days 1 & 2. But even this proved to be too much and there was further reduction to 80 mg/m2, and later to 70mg/m2. Here is one unequivocal statement from this important study, and I quote:
“The maximum tolerated dose of bendamustine in refractory CLL is 70 mg/m2 on days 1 and 2”.
We are all adults here, and not too many of us believe in the tooth fairy anymore. We all know cancer therapy is no walk in the park. Talking about toxicity of a particular drug is only one side of the equation. The other crucial information is how much of a bang for the buck did these guys get? How much of response did the patients get for all the pain and suffering and infection risk? Here is how it played out:
I have looked high and low, and this is the most detailed, up-to-date and credible paper I have seen on the subject of using bendamustine to treat relapsed CLL patients. If this drug is in your future, I strongly urge you to read the full text of the paper. Much of the more recent information out there seems to be PR plugs from the company or news services that picked up the same stuff. Just for the sake of contrast, here is a link to the Reuters take on it, almost verbatim from the company press release: Reuters article.
There were a handful of ASH 2026 abstracts dealing with the subject of bendamustine (branded “Treanda” by its owner, Cephalon, Inc.). Two were of particular note for us guys with CLL. The first abstract, given below, reported on the company-sponsored Phase III trial, comparing bendamustine with the old favorite, chlorambucil. Actually, I was kind of surprised ASH allowed this as only a poster at the conference. Typically, Phase III trials like this with a large number of patients enrolled get more prominent exposure, as full fledged oral presentations.
[2043] Bendamustine Versus Chlorambucil in Treatment-Naive Patients with B-Cell Chronic Lymphocytic Leukemia (B-CLL): Results of an International Phase III Study. Session Type: Poster Session, Board #233-II
Wolfgang Ulrich Knauf, Toshko Lissichkov, Ali Aldaoud, Raoul Herbrecht, Anna Marina Liberati, Javier Loscertales, Gunnar Juliusson, Christian Dittrich, Karlheinz Merkle
Hem./Onc., Onkologische Gemeinschaftspraxis, Frankfurt, Germany; Hematol. Transfusion Med., National Hematological Center, Sofia, Bulgaria; Hem./Onc., Onkologische Praxis, Leipzig, Germany; Hem./Onc., Hopital Universitaire Hautepierre, Strasbourg, Strasbourg, France; Oncol., Universita degli Studi, Perugia, Italy; Hematol., Hopital de la Princesa, Madrid, Spain; Hematol., University Hospital, Lund, Sweden; Onkol., Kaiser Franz Josef Spital, Vienna, Austria; Trial Development, Med.Consultant, Miesbach, Germany
Introduction: Bendamustine (BEN) is a purine analog/alkylator hybrid agent with a particular mechanisms of action that provides effective treatment for a number of hematologic and non-hematologic malignancies. It is used primarily for chemo-nave, relapsed or refractory B-CLL as well as for other types of non-Hodgkins lymphomas. The aim of this randomized phase III, open-label, multicenter study was to compare the efficacy and safety of BEN versus chlorambucil (CLB) in treatment-nave patients (pts) with B-CLL Binet stage B/C.
Patients and Methods: Pts with untreated B-CLL were randomized to receive BEN (100 mg/m2 on days 1+2) or CLB (0.8 mg/kg on days 1+15) for up to 6 treatment cycles. Primary endpoints were overall remission rate (ORR), defined as complete response (CR), nodular partial response (nPR) and partial response (PR), confirmed after 8 weeks, and progression-free survival (PFS). Secondary endpoints were duration of remission, overall survival (OS), safety, and quality of life (QoL). Follow-up was for 12 months after completion of treatment of the last patient, or until progression for pts with CR, nPR or PR and stable disease, or until death or lost to follow-up. A 5-stage, adaptive-group, sequential procedure was used with planned interim analyses to adjust the number of pts. Safety and efficacy were assessed by an Independent Data Monitoring Committee.
Results: 305 pts were randomized to receive BEN (n=156) or CLB (n=149). As 7 pts did not receive study medication, 298 pts were included in the safety analysis. At the time of this analysis, 264 pts (139 BEN; 125 CLB) were available for the efficacy analysis. For both treatment groups: median age was 64 years; 70% had Binet stage B and 30% Binet stage C disease; median number of cycles/patient was 6; median follow-up was 18.5 months. ORR was significantly higher with BEN than with CLB (68% vs 39%; p<0.0001), with a CR of 30% vs 2%, respectively. Among the subgroups with Binet stage B and C disease, ORR was 70% and 61%, respectively, with BEN, vs 47% and 22%, respectively, with CLB. Median PFS (Kaplan-Meier estimate) was 21.7 months with BEN and 9.3 months with CLB (p<0.0001), and median duration of remission was 18.9 months with BEN and 6.1 months with CLB (p<0.0001). No difference in OS was seen between groups. Toxicity of BEN was manageable and did not impair QoL when compared with CLB. Infection rates (common toxicity criteria grades III+IV) were low in both groups (5.8% BEN; 3.5% CLB).
Conclusions: BEN was significantly more effective than CLB in achieving remissions in treatment-nave pts with B-CLL Binet stage B/C; median PFS and duration of remission were also significantly longer. Furthermore, safety data indicate that BEN toxicities are manageable and the drug is well tolerated. On the basis of these results, BEN should be considered as first-line chemotherapy for patients with B-CLL Binet stage B or C.
Abstract #2043 appears in Blood, Volume 110, issue 11, November 16, 2026
Disclosure: Consultancy: Karlheinz Merkle consultant for Mundipharma. Honoraria Information: Wolfgang Knauf received honoraria from Mundipfarma. Membership Information: Wolfgang Knauf and Karlheinz Merkle are members of an advisory board of Mundipharma.
Wait just a darned minute! How come this pivotal phase III trial was done with a dosage of 100mg/m2 for two days, when the maximum tolerated dose established by the prior German CLL Study Group established 70mg/m2 for 2 days as the limit? I suppose a case can be made that these are treatment-naïve patients, and therefore more able to handle the heavy duty dosage. We hope. All the same, were these patients on any sort of prophylaxis regimen, to protect against the serious and frequent myelosuppression and infections seen in the earlier German trial? The ASH abstract is silent on this important subject. We can only hope this poster presentation will be followed up by more detailed full length papers in peer reviewed journals.
A second and obvious question is why Treanda was compared to chlorambucil in this important clinical trial. Surely chlorambucil is just a tad old-fashioned (and sets a low efficacy bar) and we have a lot better drugs available right now? The only way I can explain this choice is that framing the question in this way gets the manufacturer the answer he wants. Talk about setting up a straw man that is easy to knock down! We saw a similar approach taken recently in getting Campath approved as single agent frontline therapy for CLL patients (Campath – Jumping the Gun). In that case too, the comparison drug was chlorambucil and in both clinical trials they managed to test against a less than effective dosage of chlorambucil. I doubt many practicing hematologists/oncologists would consider 0.8mg/kg of chlorambucil twice a month as appropriate therapy or dosage for advanced CLL patients.
The abstract goes into details of response to therapy. As you would expect, bendamustine did better than good old chlorambucil on overall response rate, percentage of CRs, duration of remission, etc. But here is the kicker: “No difference was seen in overall survival between groups”! Oh well. More of you passed the test with flying colors, got that coveted “CR” on your exam; too bad you did not live any longer because of it. Sheesh. These cancer patients are a whiny bunch, there is no satisfying them.
OK, let us look at the second ASH 2026 abstract on the subject of bendamustine.
Comparing this report with the one we just finished discussing is not straightforward. This second one deals with relapsed patients — and in this case the bendamustine is goosed along with our favorite monoclonal antibody, Rituxan. Heck, every chemotherapy drug known to man works better when combined with Rituxan so it is only logical to see if this new old player behaves as expected.
[3106] Bendamustine in Combination with Rituximab (BR) for Patients with Relapsed Chronic Lymphocytic Leukemia (CLL): A Multicentre Phase II Trial of the German CLL Study Group (GCLLSG).
Session Type: Poster Session, Board #325-III
Kirsten Fischer, Stephan Stilgenbauer, Carmen D. Schweighofer, Jasmin Renschler, Raymonde Busch, Michael Kiehl, Leopold Balleisen, Michael Eckart, Anna M. Fink, Sebastian Boettcher, Matthias Ritgen, Michael Kneba, Peter Staib, Hartmut Doehner, Barbara F. Eichhorst, Michael Hallek, Clemens-Martin Wendtner
The German CLL Study Group (GCLLSG) Department of Internal Medicine I, University Hospital, Cologne, Germany; Department of Internal Medicine, University Hospital, Ulm, Germany; Institute for Medical Statistic and Epidemiology, Technical University, Munich, Germany; Department of Internal Medicine, Hospital, Frankfurt Oder, Germany; Department of Internal Medicine, Hospital, Hamm, Germany; ISP Naegelsbacherstrasse, Erlangen, Germany; Department of Internal Medicine, University Hospital, Kiel, Germany; GCLLSG, Germany
Introduction: Bendamustine has shown considerable activity in monotherapy for solid and lymphoid malignancies including CLL and has been used for more than 30 years in anti-cancer treatment. In vitro and ex vivo studies have demonstrated synergistic pro-apoptotic effects of bendamustine and the CD20 antibody rituximab (BR) in primary B-CLL cells. Encouraging clinical results have been obtained using BR combination treatment in relapsed/refractory NHL or mantle cell lymphoma. The present phase II trial represents the first study assessing efficacy and toxicity of bendamustine in combination with rituximab in pts with relapsed/refractory B-CLL.
Patients and Methods: Between March 2026 and June 2026 81 pts with relapsed/refractory B-CLL and a median number of 2 pretreatments were enrolled into this trial. Bendamustine was given at a dose of 70 mg/m2 on day 1 and 2, combined with 375mg/m2 rituximab for the first cycle and 500 mg/m2 for the second and subsequent cycles. BR treatment was administered every 28 days up to 6 courses. Blood samples were taken for molecular cytogenetics by fluorescence in situ hybridization (FISH), analysis of the IgVH mutational status and expression of ZAP70/CD38. Assessment of minimal residual disease (MRD) was evaluated in peripheral blood and bone marrow by 4-colour flow cytometry.
Results: So far data of 31 pts (median age 66 years) with a total of 126 treatment cycles are available for analysis. There was notable toxicity with 48 reported CTC grade 3/4 adverse events, particularly myelosuppression and infections: grade 3/4 anemia occurred in 6.3.%, leukopenia/neutropenia in 10.8% and thrombocytopenia in 11.9% of all courses. 6 episodes of CTC grade 3/4 infections were documented: 1 sinusitis maxillaris, 1 FUO and 1 abscess, all of them being reversible after antibiotics. However, 3 pts died due to severe infections including 2 fatal pneumonias and 1 urosepsis. One pt died due to myocardial infarction. Regarding efficacy, 23 pts were evaluable for response: The overall response rate (ORR) was 65.2% with a CR rate of 13.0% (3 pts) and a PR rate of 52.2% (12 pts). No molecular remission was observed by 4-colour cytometry. Stable disease (SD) was achieved in 26.1% (6 pts) whereas 2 pts had progressive CLL (PD, 8.7%). Responses were observed in the majority of pts with genomic aberrations 11q deletion (ORR: 4/5) and trisomy 12 (ORR: 2/3), while none of the pts with 17p deletion (ORR: 0/4) was responsive (P=0.006).
Conclusion: Bendamustine plus rituximab is an effective regimen in refractory/relapsed B-CLL. Major treatment toxicities were myelosuppression and infections. Updated data with respect to toxicity and efficacy based on the entire study population will be available for the Meeting.
Abstract #3106 appears in Blood, Volume 110, issue 11, November 16, 2026
Disclosure: No relevant conflicts of interest to declare.
In this combination trial, 81 CLL patients were recruited, all of them relapsed or refractory. In keeping with the maximum dosage guidelines laid down by the CLL German Study Group, the dosage of bendamustine was only 70mg/m2 for 2 days in each cycle. Rituxan was given at 375mg/2 for the first cycle, then increased to 500mg/m2 for subsequent cycles. Each cycle was 4 weeks, and patients went through a maximum of 6 cycles. Since bendamustine is thought to act like a combination of alkylating agent and purine analog, perhaps it is fair to compare bendamustine + Rituxan to something like FCR (fludarabine + cyclophosphamide + Rituxan).
While 13% of the patients got a “CR”, there were no molecular remission (in other words, no pcr negative responses). The overall response rate was 65%, pretty much in the same ball-park as FR and FCR type combos. But here is the eye-opener for me: out of the 31 patients on whom the abstract reported data, 3 patients died due to severe infections and another one died due to cardiac infarction. As the authors note and I quote, “notable toxicity with 48 reported CTC grade 3/4 adverse events” in just these 31 patients! For your information, grade 3-4 adverse effects are serious events, even macho men need not be ashamed to cry uncle when hit with this level adverse events. When was the last time you saw similarly scary statistics with FR or FCR therapies?
By the way, please notice that none of the patients with 17p deletions (four of them) responded to the combination of Rituxan plus bendamustine. We all know that Rituxan does not do much for these guys and it looks like adding bendamustine does not improve matters any.
Treanda is the trade name for the old drug bendamustine. With that out of the way, let us look at the details.
As I have said before, bendamustine is thought to act like a combination of purine analog and an alkylating agent. In other words, you might think the best way to evaluate this drug is to compare it against combinations such as fludarabine and cyclophosphamide. In fact, there was a hugely credible and large scale study done in Europe, which looked at the F + C combination in CLL. Surely results from that trial would have been a better comparison for bendamustine?
Looking over the German CLL Study Group paper we reviewed above, it sounds to me that bendamustine does indeed act like a combination of alkylating agent and purine analog, in both efficacy as well as toxicity. Would there have been as many deaths (early deaths in 19% of patients, life threatening infections in 12.5%) if these patients had been treated with plain old chlorambucil, instead of bendamustine?
While it is obvious that comparing the two ASH abstracts is a bit like comparing apples and oranges, I was nevertheless struck by the difference in the tone of the two abstracts. The first one was much more upbeat, saw the half full glass and felt the results justified approval of Treanda / bendamustine as frontline therapy for CLL patients. The second abstract was less optimistic and the focus was a bit more on scary deaths, infections, toxicity, etc. Call me a cynic, but I do believe money makes the world go round. Just take a quick peek at the disclosed conflicts of interest for the two abstracts. The first one is very heavy on corporate sponsored researchers, the second one cites no conflicts of interest. Beauty is in the eyes of the beholder, or to be more specific in this case, beauty can be made to fit the requirements of the guy paying the bills. Cynical? You bet. But tell me, which one of these two abstracts would you tend to trust more, when making decisions for yourself?
 Why on earth do the makers of all these “new” drugs compare their product to chlorambucil — and then proceed to give the chlorambucil in less-than-optimal dosages? We have commented on a similar approach taken by the makers of Campath: see Campath: Jumping the Gun? This seems to be a new trick that drug manufacturers have learned. In order to get past regulatory hurdles, they set up a David-versus-Goliath comparison, use an easily-knocked-down straw man such as chlorambucil, make sure the dosage for the comparison drug is less than adequate and declare victory when their drug passes this unfair and unsound comparison. In the opinion of this layperson observer, this sounds like gaming the system, nothing more and nothing less. Simply a way of getting past regulatory hurdles. Seriously folks, I think we deserve better than that.
Why on earth do the makers of all these “new” drugs compare their product to chlorambucil — and then proceed to give the chlorambucil in less-than-optimal dosages? We have commented on a similar approach taken by the makers of Campath: see Campath: Jumping the Gun? This seems to be a new trick that drug manufacturers have learned. In order to get past regulatory hurdles, they set up a David-versus-Goliath comparison, use an easily-knocked-down straw man such as chlorambucil, make sure the dosage for the comparison drug is less than adequate and declare victory when their drug passes this unfair and unsound comparison. In the opinion of this layperson observer, this sounds like gaming the system, nothing more and nothing less. Simply a way of getting past regulatory hurdles. Seriously folks, I think we deserve better than that.
Don’t get me wrong. We need all the help we can get in treating refractory CLL patients. All you have to do is read the dismal statistics we reported recently (Refractory CLL) for this group. And bendamustine has a mix of properties that might make it an interesting addition to our lineup of available drugs. But I am getting very tired of these rigged comparisons (or no comparisons at all) as companies try to get FDA approval. It is also becoming a pattern that most of these phase III trials are done in Europe (the old Eastern Europe, to be more precise), where I suppose things are done a little differently. The results are then used to convince US regulatory authorities. After all, the really big bucks are made in the US market. This is where drug companies get rich. How about doing it the right way folks, with meaningful comparisons and honest apples-to-apples assessments of efficacy and toxicity? No doubt part of the problem rests with our own regulatory agencies who may be using outdated or plain unreasonable standards.
As we have mentioned above, bendamustine is hardly new and drugs this long in the tooth rarely have patent protection. Goody, that means it will have generic competition and be easy on the wallet, right? Wrong! I fully expect that when/if it gets FDA approval for marketing in this country, you (or your insurance company) will be asked to pay the usual arm and leg for it. How come? Well, as of August 17, 2026 Treanda has been granted orphan drug status by the FDA, which means the owners get seven years of protection from competition in the U. S. market — the orphan drug exclusivity. You will also note the name Treanda is so much more trendy than bendamustine – nothing ‘musty’ about it. Kudos to the PR firm – or marketing executive – that came up with the new name.
 Most Americans love conspiracy theories. We still talk of the ‘grassy knoll’ in connection with the JFK assassination. Heck, more of us are fans of this national pastime than mere spectator sports! Here are some links to whet your appetite, make of them what you will. Commenting on the financial conflicts and/or ethical behavior of companies is beyond my expertise – but I am sure you can form your own opinions.
Most Americans love conspiracy theories. We still talk of the ‘grassy knoll’ in connection with the JFK assassination. Heck, more of us are fans of this national pastime than mere spectator sports! Here are some links to whet your appetite, make of them what you will. Commenting on the financial conflicts and/or ethical behavior of companies is beyond my expertise – but I am sure you can form your own opinions.
Philadelphia Business Journal: Cephalon settles drug marketing probe for $425M;
FDA Warning Letter to Cephalon on Provigil;
Wall Street Journal: Cephalon Drug (Fentora) Is Tied to Several Deaths;
Press Release: Connecticut Attorney General on Marketing of Fentora;
The Star-Ledger Pharmalot Blog on Cephalon and Off-label Marketing;
Task Force Report: Corporate Funding and Conflicts of Interest.
Editor's Note: Cephalon, Inc. holds exclusive rights to market and develop TREANDA in the United States. TREANDA is licensed from Astellas Pharma GmbH. Bendamustine HCl, the active ingredient in TREANDA, is marketed in Germany by Astellas' licensee, Mundipharma International Corporation Limited, under the tradename RIBOMUSTIN(R). In Germany, RIBOMUSTIN is indicated as a single-agent or in combination with other anti-cancer agents for indolent NHL, multiple myeloma, and CLL. SymBio Pharmaceuticals Ltd holds exclusive rights to develop and market bendamustine HCl in Japan and selected Asian countries. Source: Biospace.com.
Keep the scorecard handy: this lineup will probably change over time.
We would welcome a chance to discuss this agent with Cephalon and address points raised here which are of importance to the patient community.
 Enter Keywords: |
———
Disclaimer: The content of this website is intended for information only and is NOT meant to be medical advice. Please be sure to consult and follow the advice of your doctors on all medical matters.
Copyright Notice:
Copyright © 2026-2007 CLL Topics, Inc. All Rights Reserved.
All materials contained on this site are protected by United States copyright law and may not be reproduced, distributed, transmitted, displayed, published or broadcast without the prior written permission of CLL Topics, Inc. You may not alter or remove any trademark, copyright or other notice from copies of the content.
However, you may download and print material from CLLTopics.org exclusively for your personal, noncommercial use.
———
