 |
||||||||||
Date: December 27, 2026
by Chaya Venkat

The Continuing Saga of the Round-headed Kid;
Harvey's Chocolates;
Remission Managment for the Round-headed Kid;
Rituxan Road Block;
Harvey Is Back;
Strategy and Tactics.
The Usual Scenario |
Are any of you fans of the old Charles Schultz "Peanuts" cartoons?
I am. There is one cartoon that I find particularly poignant: Lucy holds the football for Charlie Brown, holding it just right for him to kick a perfect field goal. But she always pulls it away at the last moment and the eternally trusting and optimistic round-headed kid ends up flat on his back one more time. Sometimes, CLL and CLL therapies remind me of that classic bait and switch: the perfect, low toxicity and guaranteed response therapy is perpetually just around the corner, but just outside of our reach.
Today I want to write about the round-headed kids in our midst, the ones with "Bucket C" written on their bald foreheads. This is a story of one round-headed kid named Harvey who decided to take one more shot at kicking that football. Who knows, may be this time Fate will not yank it away at the last moment and he will get to kick that perfect field goal?

Harvey was trundling along, minding his own business, just happy to have a more or less indolent form of CLL. Being a bit of nerd and given to neat tabulations of all snippets of information, he was pretty sure he was one of the lucky Bucket A folks, what with his 13q deletion (best of the best!) and CD38 at a scant 18%. Life was good, or at least it was tolerable. Being the responsible sort, he went in for his annual check-up, just to make sure everything was still on track. This last time around when the results came in, it was like a kick in the stomach. You guessed right, Harvey had a "clonal evolution event". Right alongside his lovely, indolent 13q deletion clone, a new and very ugly baby clone appeared, the dreaded 11q deletion clone. And this baby was growing fast, faster than its older sibling. One quick refresher course of "What Type of CLL Do You Have?” confirmed Harvey's worst suspicions: his goose was cooked, he was no longer in the lovely and comfortable Bucket A, he faced the dread and unknown risks of Bucket C.
 |
The “Bucket-C” Kid |
Looking at him, you would not have thought this round-headed kid had what it took, but Harvey was glad he found out the bad news: ignorance is not bliss. Heck, he had just read this abstract in the latest (2003) ASH Education Book, that 11q deletion means deletion of the very important ATM gene, and people with this abnormality do not respond well to alkylating agents and purine analogs. Harvey looked them up just to be sure: some of the better known alkylating agents of importance to CLL patients are chlorambucil and cyclophosphamide, and the purine analogs fludarabine and pentostatin are still considered the gold standard by many oncologists for treating CLL patients. Boy, that was an eye-opener! Ever since his lymph nodes started getting bigger, his local oncologist had been pushing him to go with straight fludarabine therapy. If Harvey had not bothered to keep track of his FISH cytogenetics, he would never have known that with the dominance of the new 11q deleted clone, his chances of getting a really deep remission with fludarabine monotherapy were not as good as they were before the clonal evolution. All that chemo-toxicity of a DNA mutating drug would have been for less than stellar results. No, our hero had to find another way.
First things first, Harvey settled down to some heavy reading. He had to find out more about this 11q / ATM gene, why it was so darn important. (Cytogenetics of ATM and P53; The TP53 Gene). Not that he was trying to learn all the complex cytogenetic issues, just enough to make sensible decisions for his own healthcare. This is what he learned. Most chemotherapy drugs, including fludarabine, cyclophosphamide and chlorambucil are toxic to cancer cells, because the drugs cause breaks in the double strands of the DNA of the cells. When the damage is so extensive, the cell’s own internal systems throw up their hands in despair (the damage is beyond their repair capabilities) and issue the orders for the cell to kill itself (apoptosis). One very important member of that internal system is the ATM gene. With this gene missing in action, there is no one to wake up the tumor suppressor gene TP53, the one with the responsibility to tell the damaged cell to commit hara-kiri, it is allowed to continue living in its mangled and dangerously wounded fashion. In fact, some cell repair mechanisms may kick in, do a sloppy job of repair and call it a day. He looked, but could not find any "smoking gun" reference that tied 11q deletion and subsequent fludarabine therapy with higher risk of dangerous things like Richter's Transformation down the road. No one has quite connected the dots yet, it seemed, perhaps there was no connection. But it made sense to Harvey that leaving badly mangled CLL cells alive is sort of like leaving a dangerous and hurt carnivore on the loose - it is just asking for more trouble down the road.
OK, now he knew what he faced. Standard issue chemotherapy with fludarabine and the like was probably not the best choice for him. But what about the recent excitement about combinations like "RF" (Rituxan plus fludarabine) and RFC (Rituxan + fludarabine + cyclophosphamide) and other variations on that theme? (Rituxan plus Chemotherapy Combinations). There were plenty of papers out there, suggesting that many patients got much better responses when chemotherapy was combined with monoclonal therapy. But no matter how hard he looked, he could not find clear and unambiguous information on how 11q deleted folks fared with these combo regimes. Were the dregs of these clinical trials, the 10%-20% of patients who did not respond to these combos, were these mostly the unfortunate "Bucket C" folks? It was frustrating. Surely the researchers had done Interphase FISH analysis on all their patient participants ahead of time - what was so hard about breaking out the clinical trial results in terms of the specific cytogenetics? What is the point of having these sophisticated tests, if they were not used to help patients make better therapy choices? Harvey thought he would barf if he read one more learned paper that used lymph node size (measured by a ruler, no less!) as a criterion for response.
Cutting his losses, Harvey decided that in the absence of clear data proving otherwise, the prudent thing to do was to assume that if standard chemotherapy drugs are not going to work well for him, combos using them are not going to be the best choices for him either. Oh yes, he also picked up the little nugget of information that radiation was out too, 11q (ATM) or 17p (TP53) deletion meant he was not going to respond cleanly to radiation either.
Just as he was getting really depressed looking at the survival statistics for 11q deleted folks (What Type of CLL Do You Have?), he came across some encouraging news. The problem with some of the older papers (papers that are just 5 years old, for Pete's sake!) was that they were based on statistics obtained before the advent of the monoclonals like Rituxan and Campath. Harvey learned that monoclonals work by an entirely different mechanism. By targeting specific markers on the cancer cells (CD20 in the case of Rituxan and CD52 in the case of Campath), they tag the tumor cells as "foreign" and therefore dangerous. The body has nice and courteous manners where its own cells are concerned, politely inviting them to commit suicide when they are damaged too much and beyond repair. But for dastardly invaders such as bacteria, no such niceties are required. Decorous suicide is too good for these nasty things - the body uses every available means at its disposal to kill them. Obviously, the body cannot expect these rude and unmannerly invaders to commit suicide on command! Since there is no reason to expect cell suicide from the attacking hordes of bacteria, cancer cells tagged with monoclonals and looking like foreign pathogens are fair game for slaughter by any means available to the body. That was it, in a nutshell. Monoclonal antibodies like Rituxan and Campath tagged cancer cells and made them look like dangerous and "non-self" invaders, and this tricked the body into bringing on all its skills in getting rid of pathogenic invaders, not just depending on internal ATM and TP53 controls to kick in.
Harvey thanked his lucky stars that he had not been in this sticky spot 5 years ago, when the scary statistics of Dohner, et al. would have been rightly applicable to him. But here and now, he had a few more options than patients in his situation had just a few years ago. Perhaps he could still work this out.
It never rains but it pours. As he read more Rituxan and Campath articles, he realized that these drugs seem to have a problem dealing with heavy tumor loads. It is almost as if these fine-tuned drugs worked well where they did not have to deal with entrenched and hard-to-reach enemies. Rituxan and Campath seemed to do a great job cleaning out the CLL cells in the peripheral blood circulation. But Harvey was too old at this game to be fooled by that. He knew that for majority of CLL patients the real battlefields are the bone marrow and the swollen lymph nodes and spleen. More than 90% of the cancer cells live in these cozy and nurturing neighborhoods, converted to suit their malignant tastes and needs. In the midst of their kith and kin, they made babies and grew their populations - and they were harder to kill. Campath seemed a little better at clearing out the bone marrow, but even this monoclonal had problems dealing with bulky lymph nodes. As he fingered his lumpy looking jaw line, Harvey looked for better definition of "bulky". It seemed that the cut-off was lymph nodes bigger than 2cms. Chances of getting a squeaky clean response with little or no detectable residual disease were not good if patients went into Campath therapy with lymph nodes much bigger than an inch across.
To our round-headed kid it surely looked like Lucy was going to yank out that football, just like always.
To the brave and the steadfast go the rewards of life. Harvey kept looking for a way out of the box. It helped that he was not an expert, just a layperson trying to save his own life. Without the benefit of a formal education in cancer biology or immunology, his very inexperience meant he looked in places that professional experts would not think of looking. In his desperation he began to read about bone marrow transplants. A forlorn hope, since he had no brothers or sisters who could be willing and well matched donors, and his remote Chinese background (we are calling him Harvey for our convenience. Most of us cannot even pronounce his given Chinese name) meant there were few people of his ethnic persuasion in the bone marrow donor bank in the United States. Matched Unrelated Donor (MUD) allogeneic transplants had good odds only when the match between donor and recipient was quite good. As for autologous transplants, Harvey was not sure he liked the idea of the heavy duty "conditioning" therapy needed to get sufficiently clean sample of stem cells from his own bone marrow.
The hour grew late, and he was tired, depressed and scared. Lesser men would have quit right then and there, crawled into bed and pulled the covers right over their heads. But our hero was made of sterner stuff. At last his hard work was rewarded and he saw a glimmer of hope, as he read about the methods used to gather stem cells for bone marrow transplants. He pored over many mind-bendingly complicated articles, chasing down this glimmer of light. I will make it easier on you, dear reader, and give you the punch lines.
Stem cell harvesting is one of the most important steps in bone marrow transplants. Getting enough stem cells is a major goal. It has been known for many years that G-CSF (granulocyte colony stimulating factor, trade name "Neupogen") and low-dose cyclophosphamide are good at flushing out stem cells from the bone marrow, as well as other locations of the lymphatic system. Stem cells (and other cell lines as well) are tethered to the stromal cells of the bone marrow by means of tight bonds between SDF-1 on the stromal cells and CXCR4 receptors on the stem cells themselves. G-CSF cuts these apron-strings, allowing the stem cells to float loose from the bosom of the nurturing bone marrow, out into the open blood circulation, from whence they can be harvested. The interesting thing Harvey learned, (and this is the punch line so wake up dear reader and pay attention), CLL cells are also held in place in bone marrow and lymph nodes by the very same SDF-1 and CXCR4 bonds. In fact, CLL cells express much higher levels of CXCR4 than normal cells. CLL cells in circulation in the blood respond to the siren call of SDF-1 and migrate to the bone marrow. Mute that siren call, cut the apron strings, and CLL cells are likely to become aimless in their wanderings, floating out into the blood circulation.
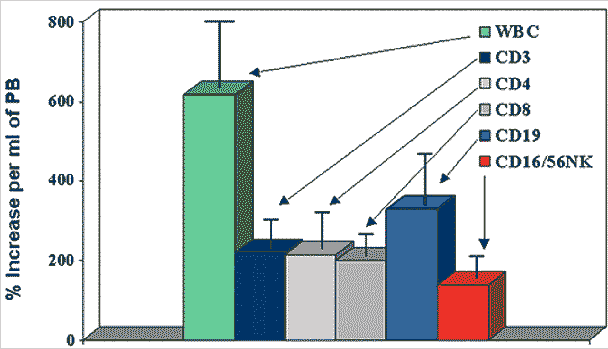
WBC Mobilization by G-CSF in Normal Donors |
I am sure you are catching my drift by now. CLL cells safely ensconced in the bone marrow and lymph nodes are hard to kill by drug administration, protected from suicidal thoughts by the constant reassuring feedback of nurse-like-cells that surround them and take care of them. But once they are flushed out of their happy homes, the rules of the game change. Here then is the tantalizing thought, how about having Rituxan or Campath out there in the peripheral blood, hungry sharks circling in the water, and at the right moment when we have enough concentration of the monoclonals in the blood, we flush out the CLL cells out of their safe hiding holes in the bone marrow and lymph nodes? Murder and mayhem will result. But since Rituxan and Campath are specific in their tagging capability, only the cells carrying the CD20 or CD52 markers respectively will be attacked. The stem cells and other cell lines flushed out of the bone marrow at the same time that the CLL cells got booted out will wander around aimlessly for a while, until the effects of the G-CSF or low dose cyclophosphamide wears out in a couple of days, then get back to their homes. The trick is that even though we are quite unspecific in booting out many cell lines out of the bone marrow and lymph nodes, combining this general “mobilization” with the very specific cell targeting capability of the monoclonals means the innocent cell lines are unharmed and allowed to return home after a period of being homeless.
G-CSF and low-dose cyclophosphamide are the present day standards for "mobilizing" (booting out) cells from the bone marrow and other lymphatic locations, techniques that are well understood and available today. Other interesting approaches are making a splash, such as the AMD3100 specific blocker of CXCR4 that we discussed a few days back (AMD3100; Adhesion, Homing and Resistance to Therapy). Unlike the growth factor G-CSF and the chemotherapy drug cyclophosphamide that have complex and multi-faceted effects, AMD3100 and similar antagonists of the CXCR4 cytokine are simple and small molecules that do one thing: they plug up the CXCR4 port, and make the CLL cell deaf, unable to hear the call of SDF-1, and unable to mate with it even if they happen to come by the bone marrow by sheer happenstance. And the other good thing about these new molecules is that their effects wear out quite quickly, allowing things to go back to normal.
Harvey began to formulate his game plan. Since he had good CD20 expression, and in fact he had responded well to Rituxan the last time he tried it, he decided he would stick with Rituxan and save Campath as a fall back option for later on. He would ask his CLL Consortium expert whether it is possible to have G-CSF administered in the right quantity and right sequence with a series of Rituxan infusions, so that he had good chance of getting the CLL cells out into the open peripheral blood, where Rituxan can do its selective cell kill. This way, he may stand a chance of getting rid of most if not all of the CLL cells, not just the few that happened to be out in the open to begin with. All this, and not too much to worry about on the deleted 11q front, since the mechanism of cell kill with monoclonals did not depend exclusively on TP53 or ATM based pathways!! He flagged for discussion the issue of whether he should try G-CSF by itself or add low dose cyclophosphamide as well, whether that would do a better job of flushing out the CLL cells. Just for the sake of completion, he also jotted down "AMD3100 ??", to remind himself to ask his CLL expert if this new drug can be sprung loose for him. It had been approved by the FDA, it actually had “orphan drug” status, but only for testing in standard bone marrow transplants. Hey, you never get anything if you don't even ask for it, right?
Way back in his college days in Boston, his then girlfriend had dragged him out to a lecture that had sounded very boring. But he was glad he had gone. There was pin drop silence in the packed standing room only auditorium as Professor Judah Folkman described his theories of angiogenesis. Harvey was never much of a biology buff, he liked the crisp clarity of engineering equations a lot better. But there was an elegance and beauty in the breathtaking simplicity of Dr. Folkman's theory of angiogenesis and he never forgot the man's brilliant insight. Cancer cells need nutrition, food and air, just like all living cells. Any collection of cancer cells bigger than a peppercorn will not survive unless it is able to jury-rig blood vessels to bring food and air to the interior cancer cells. Dr. Folkman had a novel suggestion: starve the tumor by preventing the growth of new blood vessels, and you will prevent its growth. Preventing angiogenesis may not get rid of the last cancer cell in your body. But it may prevent the cancer from growing, contain the malignancy to a few frustrated cells that cannot take over vital body functions. As a communications engineer, Harvey could understand the concept of functional stalemate. In his own area of expertise, he knew it is impossible to transmit a signal that had absolutely no noise associated with it. But as long as the signal to noise ratio was acceptable, a certain amount of noise is something that one can learn to live with, and soon learn to ignore completely.

It feels good |
Since this was his lucky night and he was on a roll, he continued his readings the whole night through. Just as dawn was breaking, he finished writing up his personal CLL therapy protocol wish-list. To the G-CSF and Rituxan combination he added a chemopreventive and anti-angiogenesis chaser, insurance to protect him from any angiogenetic effects of the growth factor G-CSF. There was some recent concern that all growth factors, not just Epoetin (The Dark Side of Epoetin), can cause a burst of angiogenesis activity and thereby subvert remissions. Harvey penciled in green tea extracts (Do You Like Drinking Green Tea?) and Celebrex (NF?B - Nuclear Factor Kappa B) as a potent anti-angiogenesis regime after the completion of the Rituxan therapy. Sometimes it helps being Chinese, he thought wryly. He actually liked the taste of green tea! And who knows, down the road in few years if he had to repeat the process, newer versions of AMD3100 and anti-angiogenesis drugs like Avastin and Endostatin may become commercially available choices for him. Even if he stopped responding to Rituxan, he had Campath as a fallback option to use in the same “flush ‘em out first and then kill ‘em dead” approach.
Satisfied with the results of his search and not worried anymore, our hero logged off his computer. He rang up his office, called in sick for the day and went to bed to sleep soundly for the first time since he got his latest FISH results. See, I told you this story had a happy ending.

Readers who are interested in reading the full articles described by the first and second abstracts cited below should write to us at  . The third citation contains a link to the full-text article (free) in the 2026 ASH Education Book, with over 150 abstract citations, many of them with links to other free full-text articles.
. The third citation contains a link to the full-text article (free) in the 2026 ASH Education Book, with over 150 abstract citations, many of them with links to other free full-text articles.
Rev Clin Exp Hematol. 2026 Mar;4(1):48-72.
Genetic features of B-cell chronic lymphocytic leukemia.
Stilgenbauer S, Lichter P, Dohner H.
Department of Internal Medicine III, University of Ulm, Germany.
The genetic features of B-cell chronic lymphocytic leukemia (CLL) are currently being reassessed by molecular cytogenetic techniques such as fluorescence in situ hybridization (FISH). Conventional cytogenetic studies by chromosome banding are difficult in CLL mainly because of the low in vitro mitotic activity of the tumor cells, which leads to poor quantity and quality of metaphase spreads. Molecular genetic analyses are limited because candidate genes are known for only a few chromosomal aberrations that are observed in CLL. FISH was found to be a powerful tool for the genetic analysis of CLL as it overcomes both the low mitotic activity of the CLL cells and the lack of suitable candidate genes for analysis. Using FISH, the detection of chromosomal aberrations can be performed at the single cell level in both dividing and non-dividing cells, thus circumventing the need of metaphase preparations from tumor cells. Probes for the detection of trisomies, deletions and translocation breakpoints can be applied to the regions of interest with the growing number of clones available from genome-wide libraries. Using the interphase cytogenetic FISH approach with a disease specific set of probes, chromosome aberrations can be found in more than 80% of CLL cases. The most frequently observed abnormalities are losses of chromosomal material, with deletions in band 13q14 being the most common, followed by deletions in 11q22-q23, deletions in 17p13 and deletions in 6q21. The most common gains of chromosomal material are trisomies 12q, 8q and 3q. Translocation breakpoints, in particular involving the immunoglobulin heavy chain locus at 14q32, which are frequently observed in other types of non-Hodgkin's lymphoma, are rare events in CLL. Genes affected by common chromosome aberrations in CLL appear to be p53 in cases with 17p deletion and ataxia telangiectasia mutated (ATM), which is mutated in a subset of cases with 11q22-q23 aberrations. However, for the other frequently affected genomic regions, the search for candidate genes is ongoing. In parallel, the accurate evaluation of the incidence of chromosome aberrations in CLL by FISH allows the correlation of genetic abnormalities with clinical disease manifestations and outcome. In particular, 17p abnormalities and deletions in 11q22-q23 have already been shown to be among the most important independent prognostic factors identifying subgroups of patients with rapid disease progression and short survival. In addition, deletion 17p has been associated with resistance to treatment with purine analogs. Therefore, genetic abnormalities may allow a risk assessment for individual patients at the time of diagnosis, thus giving the opportunity for a risk-adapted management.
PMID: 11486330
____________
Leukemia. 2026 Jan;18(1):1-10.
Homing and mobilization of hematopoietic stem cells and hematopoietic cancer cells are mirror image processes, utilizing similar signaling pathways and occurring concurrently: circulating cancer cells constitute an ideal target for concurrent treatment with chemotherapy and antilineage-specific antibodies.
Gazitt Y.
University of Texas Health Science Center, San Antonio, TX.
Adhesion molecules and stromal cell-derived factor-1 (SDF-1)/CXCR4 signaling play key role in homing and mobilization of hematopoietic progenitor (HPC) and hematopoietic cancer clonogenic cells (HCC). High expression of VLA-4 is required for homing of HPC and HCC, whereas downregulation of these molecules is required for successful mobilization of HPC and HCC. Upregulation and activation of the SDF-1/CXCR4 signaling is required for homing of HPC and HCC, whereas disruption of the SDF-1 signaling is required for mobilization of HPC and HCC. Hence, mobilizations of HPC and HCC occur concurrently. It is proposed that drug resistance evolves as a result of repeated cycles of chemotherapy. Following each cycle of chemotherapy, HCC lose adhesion molecules and SDF-1 signaling. Surviving cells, released from tumor sites, circulate until re-expression of adhesion molecules and CXCR4 occurs, then homing to stroma of distal tissues occurs. Cytokines secreted by cells in the new microenvironment induce proliferation and drug resistance of HCC. This process is amplified in each cycle of chemotherapy resulting in disease progression. A novel model for treatment is proposed in which circulating HCC are the target for clinical intervention, and concurrent treatment with chemotherapy and antilineage-specific antibodies will result in abrogation of the 'vicious cycle' of conventional anticancer therapy.
PMID: 14574330
____________
Full-text Article in 2026 ASH Education Book
Stem Cell Mobilization
Michele H. Cottler-Fox, Tsvee Lapidot, Isabelle Petit, Orit Kollet, John F. DiPersio, Dan Link and Steven Devine
Successful blood and marrow transplant (BMT), both autologous and allogeneic, requires the infusion of a sufficient number of hematopoietic progenitor/stem cells (HPCs) capable of homing to the marrow cavity and regenerating a full array of hematopoietic cell lineages in a timely fashion. At present, the most commonly used surrogate marker for HPCs is the cell surface marker CD34, identified in the clinical laboratory by flow cytometry. Clinical studies have shown that infusion of at least 2 x 106 CD34+ cells/kg recipient body weight results in reliable engraftment as measured by recovery of adequate neutrophil and platelet counts approximately 14 days after transplant. Recruitment of HPCs from the marrow into the blood is termed mobilization, or, more commonly, stem cell mobilization.
In Section I, Dr. Tsvee Lapidot and colleagues review the wide range of factors influencing stem cell mobilization. Our current understanding focuses on chemokines, proteolytic enzymes, adhesion molecules, cytokines and stromal cell-stem cell interactions. On the basis of this understanding, new approaches to mobilization have been designed and are now starting to undergo clinical testing.
In Section II, Dr. Michele Cottler-Fox describes factors predicting the ability to mobilize the older patient with myeloma. In addition, clinical approaches to improving collection by individualizing the timing of apheresis and adjusting the volume of blood processed to achieve a desired product are discussed. Key to this process is the daily enumeration of blood CD34+ cells. Newer methods of enumerating and mobilizing autologous blood HPCs are discussed. In Section III, Dr. John DiPersio and colleagues provide data on clinical results of mobilizing allogeneic donors with G-CSF, GM-CSF and the combination of both as relates to the number and type of cells collected by apheresis. Newer methods of stem cell mobilization as well as the relationship of graft composition on immune reconstitution and GVHD are discussed.
__________
Genetic Aberrations of TP53 Predicts Response to Fludarabine as First Line Therapy in Patients with Chronic Lymphocytic Leukemia on Advanced Stage Rai III/IV.
Session Type: Poster Session 647-II
Maria D. Odero, Mikel Valganon, Xabier Agirre, Jose P. Roman, Idoya Lahortiga, Araceli Rubio-Martinez, Daniel Rubio-Felix, Francisco J. Novo, Pilar Giraldo, Maria J. Calasanz
Genetics, University of Navarra, Pamplona, Navarra, Spain; Fundacion estudio Hematologia y Hemoterapia (FEHHA), Zaragoza, Spain
Fludarabine (FLD) in patients with advanced CLL induces higher remission rates and longer progression free survival as first line therapy than chlorambucil or CAP. Genetic aberration that modified the expression of TP53 (deletions and mutations) are independent poor prognostic factors in CLL. Hypermethylation of the promoter regions of some tumor suppressor genes has been associated with transcriptional silencing and tumor progression. We report the genetic analysis of a group of 43 patients with CLL Rai stage III/IV treated with FLD as first line therapy (25mg/m2 IV daily 5 days) every 4 weeks, 4-6 courses until reaching response. The median age at diagnosis was 66.7 years, 26 males and 17 females. We have analyzed biological features with prognostic significance, cytogenetic aberrations, TP53 abnormalities, and the presence of somatic hypermutation of IgVH, in order to assess the frequency and clinical relevance of these features, and the relation with rates of response to FLD on advance stages of CLL. We performed FISH using the probes RPCI-11 241D13 and 415G10 (11q22.3), and LSI D13S25 (13q14), LSI TP53 (17p13.1) and D12Z3 (Vysis). We analyzed the methylation at CpG and CC(A/T)GG sites of the TP53 promoter region. Methylation of CC(A/T)GG sites represent a type of epigenetic marker that could impact transcription by altering DNA-protein complex formation. DNA-methylation was analyzed using restriction enzyme digestion followed by PCR. The association among the presence of TP53 alterations, mutational status of the IgVH genes, cytogenetic aberrations and the response to FLD of our patients, was studied using contingence tables followed by chi square or Fisher exact test. Response of 34 patients was evaluated according to the modified NCI working group criteria for CLL (9 were NV). Seventeen patients (17/34, 50%) achieved CR and 12 (12/34, 35%) PR, with an OR rate of 85% (15% of failures). We detected cytogenetic aberrations in 84% of patients (36/43), 20 (56%) had two or more abnormalities, according to previous studies that correlate the presence of more than one aberration with an advance stage of the disease. Monoallelic loss of D13S25 was the most frequent aberration (63%) followed by del(11)(q22.3) (33%), del(17)(p13.3) (26%) and trisomy 12 (21%). Only 8 cases (35%) had 13q- as the sole abnormality. We detected somatic mutations of IgVH genes (less than 98% homology) in 40% of patients. Ten patients (10/41, 25%) had methylation in the promoter region of TP53, and 3 of these also presented del(17p). Moreover, a high frequency of poor prognosis features as del(17)(p13.1), del(11)(q22.3) and no mutated IgVH genes was detected, if comparing with other series that include patients on early stage of CLL. We only found a significative correlation between the course of the disease and the presence of abnormalities on TP53. Comparing the frequency of patients with del(17p) and/or TP53 methylation versus other variables studied, we observed a significantly different non response rates in the first group (p=0,007). Cytogenetic abnormalities such as del(17)(p13) and del(11)(q22-q23) predict rapid disease progression and inferior survival in CLL. We report the impact of del(17p) and/or TP53 methylation in the resistance to treatment with FLD. Aberrations on TP53 could predict the bad response to FLD and could be used to discard purine analogues as first line therapy in patients with advanced stages of CLL.
Abstract #2476, Blood, Volume 102, issue 11, November 16, 2026
________________
Cancer Res. 2026 Jan 1;63(1):36-8.
Interphase cytogenetic abnormalities in chronic lymphocytic leukemia may predict response to rituximab.
Byrd JC, Smith L, Hackbarth ML, Flinn IW, Young D, Proffitt JH, Heerema NA.
The Division of Hematology-Oncology, Ohio State University, Columbus, OH.
Select cytogenetic abnormalities such as del(17)(p13.1) and del(11)(q22-q23)predict rapid disease progression and inferior survival in chronic lymphocytic leukemia (CLL). We sought to determine the impact of the four most common interphase cytogenetic abnormalities in 28 CLL patients relative to response to three-times-a-week rituximab therapy. Abnormalities were noted in 25 of the 28 patients to include del(13)(q14.3) [n = 16 (57%)], del(11)(q22.3) [n = 10 (36%)], +12 [n = 6 (21%)], del(17)(p13.1) [n = 5 (18%)], and normal [n = 3 (11%)]. Only a minority of each of these occurred as sole abnormalities. To categorize patients into one specific group, we used the hierarchical order del(17)(p13.1) > del(11)(q22.3) > trisomy 12 > del(13)(q14.3) to prioritize. Response to rituximab was noted to vary by cytogenetic group: del(17)(p13.1), 0% [n = 5]; del(11)(q22.3), 66% [n = 9]; del(13)(q14.3), 86% [n = 7]; +12, 25% [n = 4], and normal, 0% [n = 3]. Response was significantly lower (P = 0.05) in patients with del(17)(p13.1) as compared with those with other abnormalities. These data suggest that interphase cytogenetics in CLL may be predictive of a response to rituximab therapy and provide support for additional studies validating risk-adapted therapy in this disease.
PMID: 12517774
____________
N Engl J Med. 2026 Aug 8;347(6):452-3.
Campath-1H-induced complete remission of chronic lymphocytic leukemia despite p53 gene mutation and resistance to chemotherapy.
Stilgenbauer S, Dohner H.
PMID: 12167696
____________
J Clin Oncol. 1997 Apr;15(4):1567-74.
Phase II multicenter study of human CD52 antibody in previously treated chronic lymphocytic leukemia. European Study Group of CAMPATH-1H Treatment in Chronic Lymphocytic Leukemia.
Osterborg A, Dyer MJ, Bunjes D, Pangalis GA, Bastion Y, Catovsky D, Mellstedt H.
Department of Oncology (Radiumhemmet), Karolinska Hospital, Stockholm, Sweden.
PURPOSE: CAMPATH-1H is a human immunoglobulin G1 (IgG1) anti-CD52 monoclonal antibody (MAb) that binds to nearly all B- and T-cell lymphomas and leukemias. We report the results of a multicenter phase II trial that used CAMPATH-1H in previously chemotherapy-treated patients with chronic lymphocytic leukemia (CLL).
MATERIALS AND METHODS: Twenty-nine patients who had relapsed after an initial response (n = 8) or were refractory (n = 21) to chemotherapy were treated with CAMPATH-1H administered as a 30-mg 2-hour intravenous (IV) infusion thrice weekly for a maximum period of 12 weeks.
RESULTS: Eleven patients (38%) achieved a partial remission (PR) and one (4%) a complete remission (CR) (response rate, 42%; 95% confidence interval [CI], 23% to 61%). Three of eight patients (38%) with a relapse and nine of 21 refractory patients (43%) responded to CAMPATH-1H therapy. CLL cells were rapidly eliminated from blood in 28 of 29 patients (97%). CR in the bone marrow was obtained in 36% and splenomegaly resolved completely in 32%. Lymphadenopathy was normalized in only two patients (7%). The median response duration was 12 months (range, 6 to 25+). World Health Organization (WHO) grade IV neutropenia and thrombocytopenia developed in three (10%) and two patients (7%), respectively. Neutropenia and thrombocytopenia recovered in most responding patients during continued CAMPATH-1H treatment. Lymphopenia (< 0.5 x 10(9)/L) occurred in all patients. Two patients had opportunistic infections and four had bacterial septicemia.
CONCLUSION: CAMPATH-1H had significant activity in patients with advanced and chemotherapy-resistant CLL. The most pronounced effects were noted in blood, bone marrow, and spleen. Preferential clearance of blood may allow harvesting of uncontaminated blood stem cells for use in high-dose chemotherapy protocols.
PMID: 9193354
___________
Cancer Res. 2026 Jun 1;62(11):3106-12.
CXCR4 neutralization, a novel therapeutic approach for non-Hodgkin's lymphoma.
Bertolini F, Dell'Agnola C, Mancuso P, Rabascio C, Burlini A, Monestiroli S, Gobbi A, Pruneri G, Martinelli G.
Divisions of Hematology-Oncology, Experimental Oncology-IFOM Institute of Molecular Oncology, European Institute of Oncology, via Ripamonti 435, 20261 Milan, Italy.
The chemokine stromal cell-derived factor-1 (CXCL12/SDF-1) and its monogamous receptor CXCR4 are involved in trafficking of B cells and hematopoietic progenitors. CXCR4 expression was found in the large majority of non-Hodgkin's lymphoma (NHL) cell lines and primary cells, and CXCR4 neutralization by monoclonal antibodies had profound in vitro effects on NHL cells including inhibition of transendothelial/stromal migration, enhanced apoptosis, decreased proliferation, and inhibition of pseudopodia formation. In a nonobese diabetes/severe combined immunodeficiency (NOD/SCID) mouse model of human high-grade NHL, CXCR4 neutralization had an impressive efficacy. In a first tumor-challenge trial, CXCR4 neutralization of Namalwa cells injected i.p. delayed tumor growth and reduced tumor weight. In a second tumor-challenge trial, NOD/SCID mice received Namalwa cells i.v. All of the controls died of neoplasia within day 36, whereas 83% of mice injected with cells incubated with anti-CXCR4 were still alive and disease-free >150 days after transplant. The crucial role of CXCR4 in tumor cell extravasation was confirmed by the finding that CXCR4 neutralization before i.v. injection of Namalwa cells in NOD/SCID mice increased the number of cancer cells circulating 24 h after injection. In additional preclinical trials, the therapeutic effect of anti-CXCR4 antibodies was evaluated in mice bearing Namalwa cells injected 3 days before. Tumor growth was abrogated in the majority of treated mice and significantly delayed in the remaining group. Taken together, these data support clinical studies on CXCR4 neutralization in NHL patients by monoclonal antibodies or CXCR4 antagonists.
PMID: 12036921
____________
Eur J Haematol. 2026 May;64(5):323-32.
Stromal cells in lymph nodes attract B-lymphoma cells via production of stromal cell-derived factor-1.
Arai J, Yasukawa M, Yakushijin Y, Miyazaki T, Fujita S.
First Department of Internal Medicine, Ehime University School of Medicine, Shigenobu, Japan.
Stromal cell-derived factor-1 (SDF-1) is a chemokine produced by bone marrow stromal cells which plays an important role in B-lymphopoiesis and the homing of hematopoietic stem cells to the bone marrow. In the present study, we investigated the role of SDF-1 and its receptor, CXCR4, in the chemotactic interaction between non-Hodgkin B-lymphoma cells and lymph node stromal cells. SDF-1 mRNA was abundantly expressed in stromal cells isolated from the lymph nodes of patients with malignant lymphoma. All B-lymphoma cells freshly isolated from these patients and most laboratory B-lymphoma cell lines, including follicular, diffuse large, and Burkitt's lymphoma cells, expressed surface CXCR4 and migrated in the presence of recombinant human SDF-1alpha. Chemotaxis assays revealed that CXCR4-positive (but not CXCR4-negative) B-lymphoma cells migrated towards lymph node stromal cells, and this migration was almost completely inhibited by the addition of anti-CXCR4 monoclonal antibody to the lymphoma cells or of anti-SDF-1 neutralizing antibody to the culture supernatant of the stromal cells. Down-regulation of surface CXCR4 was detected in B-lymphoma cells which migrated towards the stromal cells but not in those which showed no migratory response. In addition, contact between the lymphoma cells and the stromal cells resulted in down-regulation of surface CXCR4 on the lymphoma cells. These data strongly suggest that SDF-1/CXCR4 is the main chemokine system involved in the chemotactic interaction between B-lymphoma cells and lymph node stromal cells.
PMID: 10863978
____________
Blood. 1999 Dec 1;94(11):3658-67.
Chronic lymphocytic leukemia B cells express functional CXCR4 chemokine receptors that mediate spontaneous migration beneath bone marrow stromal cells.
Burger JA, Burger M, Kipps TJ.
Department of Medicine, the Division of Hematology/Oncology, University of California, San Diego, La Jolla, CA.
Chemokines play a central role for lymphocyte trafficking and homing. The mechanisms that direct the tissue localization of B cells from patients with chronic lymphocytic leukemia (B-CLL) are unknown. We found that CLL B cells express functional CXCR4 receptors for the chemokine stromal cell-derived factor-1 (SDF-1), as demonstrated by receptor endocytosis, calcium mobilization, and actin polymerization assays. Moreover, CLL B cells displayed chemotaxis to this chemokine that could be inhibited by monoclonal antibodies (MoAbs) against CXCR4, pertussis toxin, or Wortmannin, a phosphatidylinositol 3-kinase inhibitor. That this chemotaxis may be involved in the homing of CLL cells is argued by studies in which CLL B cells were cocultured with a murine marrow stromal cell line that secretes SDF-1. Within 2 hours, CLL B cells spontaneously migrated beneath such stromal cells in vitro (pseudoemperipolesis). This migration could be inhibited by pretreatment of CLL B cells with anti-CXCR4 MoAbs, SDF-1alpha, or pertussis-toxin. Furthermore, we noted strong downmodulation of CXCR4 on CLL B cells that migrated into the stromal cell layer. These findings demonstrate that the chemokine receptor CXCR4 on CLL B cells plays a critical role for heterotypic adherence to marrow stromal cells and provide a new mechanism to account for the marrow infiltration by neoplastic B cells.
PMID: 10572077
____________
Cancer Res. 2026 Aug 1;63(15):4342-6.
Maximum tolerable dose and low-dose metronomic chemotherapy have opposite effects on the mobilization and viability of circulating endothelial progenitor cells.
Bertolini F, Paul S, Mancuso P, Monestiroli S, Gobbi A, Shaked Y, Kerbel RS.
Division of Hematology-Oncology, Department of Medicine, IFOM-Fondazione Italiana per la Ricerca sul Cancro, Institute of Molecular Oncology, European Institute of Oncology, via Ripamonti 435, 20261 Milan, Italy.
There is growing evidence that vasculogenesis (progenitor cell-derived generation of new blood vessels) is required for the growth of some neoplastic diseases. Here we show that the administration of cyclophosphamide (CTX) at the maximum tolerable dose with 21-day breaks or at more frequent low-dose (metronomic) schedules have opposite effects on the mobilization and viability of circulating endothelial progenitors (CEPs) in immunodeficient mice bearing human lymphoma cells. Animals treated with the maximum tolerable dose CTX experienced a robust CEP mobilization a few days after the end of a cycle of drug administration, and tumors rapidly became drug resistant. Conversely, the administration of metronomic CTX was associated with a consistent decrease in CEP numbers and viability and with more durable inhibition of tumor growth. Our findings suggest that metronomic low-dose chemotherapy regimens are particularly promising for avoiding CEP mobilization and, hence, to potentially reduce vasculogenesis-dependent mechanisms of tumor growth.
PMID: 12907602
____________
Blood. 2026 Oct 15;102(8):2728-30. Epub 2026 Jul 10.
Mobilization of hematopoietic progenitor cells in healthy volunteers by AMD3100, a CXCR4 antagonist.
Liles WC, Broxmeyer HE, Rodger E, Wood B, Hubel K, Cooper S, Hangoc G, Bridger GJ, Henson GW, Calandra G, Dale DC.
Department of Medicine, University of Washington, HSB AA-522, Box 356422, Seattle, WA.
Stromal cell-derived factor 1 (SDF1/CXCL12) and its cognate receptor, CXCR4, play key regulatory roles in CD34+ cell trafficking. We investigated whether AMD3100, a selective CXCR4 antagonist, could mobilize hematopoietic progenitor cells from marrow to peripheral blood in healthy human volunteers. Initially, 10 persons each received a single dose of AMD3100 (80 microsubcutaneously), which induced rapid, generalized leukocytosis associated with an increase in peripheral blood CD34+ cells, representing pluripotent hematopoietic progenitors by in vitro colony-forming unit assays, from 3.8 +/- 0.5/microL to 20.7 +/- 3.5/microL at 6 hours. Subsequent dose-response studies showed a maximum increase in circulating CD34+ cells from 2.6 +/- 0.3/microL to 40.4 +/- 3.4/microL at 9 hours after 240 micro/kg AMD3100. Serial administration of AMD3100 (80 microg/kg/d for 3 days) resulted in consistent, reversible increases in peripheral blood CD34+ cells. AMD3100 was well tolerated and caused only mild, transient toxicity. These findings suggest potential clinical application of AMD3100 for CD34+ cell mobilization and collection for hematopoietic stem cell transplantation.
PMID: 12855591
____________
CXCR4 Chemokine Receptor Antagonists Inhibit Activation, Migration, and Survival of Chronic Lymphocytic Leukemia B Cells in Response to Stromal Cell-Derived Factor-1 (SDF-1/CXCL12).
Session Type: Poster Session
Meike Burger, Tanja Hartmann, Nobutaka Fujii, Thomas J. Kipps, Jan A. Burger
Department of Medicine, Freiburg University Hospital, Freiburg, Germany; Graduate School of Pharmaceutical Sciences, Kyoto University, Kyoto, Japan; Division of Hematology/Oncology, University of California, San Diego, CA.
Chronic lymphocytic leukemia (CLL) is characterized by an accumulation of mature, functionally incompetent B-lymphocytes in blood, secondary lymphoid tissues, and the marrow. CLL B cells express high levels of the CXCR4 chemokine receptor (CD184) that direct leukemia cell chemotaxis in vitro. Marrow stromal cells constitutively secrete high amounts of CXCL12, which is the natural ligand for CXCR4, and thereby can attract CLL B cells. In vitro, CLL cells are rescued from apoptosis by contact with stromal or nurselike cells. The capacity of these accessory cells to protect leukemia cells from apoptosis is mediated, at least in part, by CXCL12. The CXCR4-CXCL12 axis therefore may represent a new therapeutic target in CLL. We evaluated the effects of the most active CXCR4-specific antagonists T140, TC14012, and TN14003 to inhibit CXCL12 responses in CLL cells. These inhibitors are small peptides initially developed to inhibit infection by T-tropic (X4) HIV-1 virus that utilizes CXCR4 together with CD4 for cellular entry. CLL cells pre-treated with T140 or its derivates displayed a dose-dependent inhibition of actin polymerisation, chemotaxis, and migration beneath marrow stromal cells (pseudoemperipolesis) in response to CXCL12. Furthermore, TC14012 and TN14003 antagonized the anti-apoptotic effect of synthetic CXCL12 and stromal cell-mediated protection of CLL cells from undergoing spontaneous apoptosis. Furthermore, we found that culture of CLL cells on marrow stromal cells protected CLL cells from undergoing apoptosis in response to dephosphorylated fluarabine (arabinosyl-2-fluoroadenine/F-ara-A). CXCR4 antagonists effectively inhibited stromal cell-mediated protection from F-ara-A-induced apoptosis. Using western blotting, we noticed that CLL cells pre-treated with CXCR4 antagonists displayed a strong inhibition of p44/42 MAP kinase- (ERK1/2) and Akt-activation in response to CXCL12.
These findings demonstrate that CXCR4 blocking agents can effectively antagonize CXCL12 induced migratory responses, protection from spontaneous or F-ara-A induced apoptosis, and signaling in CLL cells. As such, T140 and its derivates may be useful therapeutic tools to interfere with CXCL12 survival signals in the microenvironmental regulation of CLL cell survival.
Abstract #1585 Blood, Volume 102, issue 11, November 16, 2026
____________
Blood. 2026 Jul 1;96(1):282-7.
Endostatin, an antiangiogenic drug, induces tumor stabilization after chemotherapy or anti-CD20 therapy in a NOD/SCID mouse model of human high-grade non-Hodgkin lymphoma.
Bertolini F, Fusetti L, Mancuso P, Gobbi A, Corsini C, Ferrucci PF, Martinelli G, Pruneri G.
Divisions of Hematology-Oncology, Experimental Oncology, and Pathology-Laboratory Medicine, IRCCS European Institute of Oncology, Milan, Italy.
Both chemotherapy and chimeric anti-CD20 monoclonal antibodies are effective agents against B-cell non-Hodgkin lymphoma (NHL). However, patients achieving remission are at risk of relapse. To evaluate the effect of the antiangiogenic drug endostatin used alone and after the administration of cyclophosphamide (CTX) or the anti-CD20 antibody rituximab, we generated a new model of human NHL by transplanting Namalwa cells intraperitoneally into nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice. First, we determined the most effective treatment schedule for the drugs assessed. When administered alone, CTX (3 courses of 75 mg/kg of body weight given intraperitoneally), rituximab (3 courses of 25 mg/kg given intraperitoneally), and endostatin (5 courses of 50 microg given subcutaneously) delayed tumor growth, and CTX was the most effective in controlling bulky disease. When given after chemotherapy or immunotherapy, endostatin effectively induced tumor stabilization. When mice given CTX or rituximab on days 3, 5, and 7 after transplantation were randomly assigned to receive endostatin or phosphate-buffered saline on days 15 to 19, tumor growth was prevented in endostatin-treated mice as long as the drug was administered. Furthermore, administration of endostatin on days 25 to 29 after tumor regrowth still induced significant tumor regression, whereas CTX and rituximab were not effective. The specific antiangiogenic action of endostatin was confirmed by in vitro and in vivo studies indicating that the drug inhibited proliferation and induced apoptosis of endothelial (but not of NHL) cells. In conclusion, sequential administration of chemotherapy and endostatin seems promising for treating bulky NHL, and the less toxic sequential administration of rituximab and endostatin is promising for treating limited disease.
PMID: 10891463
____________
Cancer Res. 2026 Dec 1;63(23):8408-13.
Synergistic effect of metronomic dosing of cyclophosphamide combined with specific antitumor immunotherapy in a murine melanoma model.
Hermans IF, Chong TW, Palmowski MJ, Harris AL, Cerundolo V.
Tumour Immunology Unit, Molecular Oncology Laboratory, Cancer Research UK, University of Oxford, Weatherall Institute of Molecular Medicine, John Radcliffe Hospital, Oxford, United Kingdom.
Immunotherapy could be combined with conventional chemotherapeutic modalities aimed at reducing tumor burden. Such combination therapy may be most useful when "metronomic" doses of antineoplastic drugs are used, thereby potentially avoiding some of the immunosuppressive effects of these drugs. Recent studies have shown that some conventional antineoplastic drugs can be exploited for antiangiogenic capacities, a strategy that requires drugs to be administered at regular intervals. We therefore investigated whether such metronomic therapy with the alkylating agent cyclophosphamide (CTX) could be effectively combined with immunotherapy eliciting tumor-reactive CTLs. An immunization protocol using injection of recombinant DNA followed by injection of recombinant modified vaccinia virus Ankara strain was used to initiate a specific CTL response in mice capable of providing resistance to challenge with the murine melanoma B16.F10. Combining this immunotherapeutic regime with metronomic delivery of CTX resulted in antitumor activity that was dramatically enhanced over either treatment administered alone and was also significantly greater than combining immunotherapy with CTX administered by a maximum tolerated dose regime. Whereas both metronomic and maximum tolerated dose delivery of CTX did cause deletion of proliferating tumor-specific CTLs in the blood, this deletion occurred with slower kinetics with the metronomic schedule. Further analysis showed that metronomic CTX treatment did not delete cells with low expression of CD43, a "memory" phenotype, and that these cells maintained potent restimulatory capacity. The combination of immunotherapy and metronomic CTX therapy may be well suited to clinical management of cancer.
PMID: 14679003
____________
If you are curious about how Harvey goes about implementing his ideas and what kind of results he obtains with them, you may want to read the sequel to this story in The Continuing Saga of the Round-headed Kid.
 Enter Keywords: |
———
Disclaimer: The content of this website is intended for information only and is NOT meant to be medical advice. Please be sure to consult and follow the advice of your doctors on all medical matters.
Copyright Notice:
Copyright © 2026-2007 CLL Topics, Inc. All Rights Reserved.
All materials contained on this site are protected by United States copyright law and may not be reproduced, distributed, transmitted, displayed, published or broadcast without the prior written permission of CLL Topics, Inc. You may not alter or remove any trademark, copyright or other notice from copies of the content.
However, you may download and print material from CLLTopics.org exclusively for your personal, noncommercial use.
———
