 |
||||||||||
Date: June 23, 2026
by Chaya Venkat
Related Article:
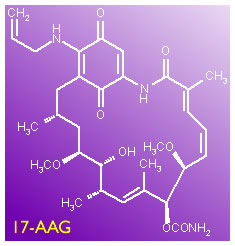
17AAG;
17AAG Overview.
In an article we published 2 days ago (17AAG) I said I was going to look into this Hsp90 inhibitor business, involving an antibiotic called 17-AAG that might be an interesting drug candidate. Well, here is the hot-off-the-presses scoop on the subject. This is our review of a brand new-yet-to-be-published article in Blood by a couple of our favorite researchers, Dr. Kipps and Dr. Castro of UCSD, as well as a very interesting multi-center clinical trial using this drug for late stage CLL patients. The trial is recruiting volunteers even as you read this article. How is that for service - fast enough for you?
 |
 |
17-AAG has become a very high profile drug, and the latest paper in Blood (the abstract is given below, write to us if you want to get hold of the full text version) gives compelling arguments for why it may become a very important drug, especially for poor prognostic, ZAP-70 positive CLL patients. In 17AAG, we discussed the mechanism of how Hsp90 acts, why cancer cells are particularly dependent on it, and therefore how blocking its function may attack the little buggers at their point of vulnerability. I will not be going into the detailed explanations again, if you are interested in this drug (and you should be!) please read the earlier article.
Unless you were just diagnosed yesterday and have not had a chance to proceed far in your reading, or you believe ostriches have the right attitude in dealing with unpleasant things, you have surely heard of ZAP-70. In addition to IgVH gene mutation status, ZAP-70 has become an important prognostic indicator (What Type of CLL Do You Have?; Prognostic and Monitoring Test Packages). Patients with a high level of ZAP-70 expression on their CLL cells (20% is the cut-off point used by many labs) have poorer prognosis than those with lower levels of ZAP-70 expression. Mind you, there is still no robust consensus on exactly how to measure ZAP-70, or even what the cut-off point ought to be. Until that happens, be sure to keep the salt shaker handy as you contemplate your ZAP-70 results. But there is broad consensus that high ZAP-70 expression goes hand in hand with a rapidly proliferating disease. Most often (but not always) high ZAP-70 expression is seen in patients in the high-risk IgVH gene unmutated group.
The reason why the ZAP-70 protein is important is that it is a fragment of the B-cell receptor (“BCR”), the port through which B-cells receive signals to go forth and multiply. CLL cells with a high level of expression of ZAP-70 are only too happy to receive and act upon the signal to have more babies. This is at the heart of why ZAP-70 is important - it facilitates active signaling through the B-cell receptor which in turn drives rapid CLL proliferation. Shutting down ZAP-70 and therefore interfering with the BCR signaling port may be good thing to do.
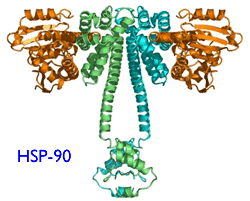
Like all proteins, ZAP-70 is a huge molecule with lots of arms and legs to it. For it to work right, this protein has to be folded just right, into exactly the right three-dimensional shape. If it is not folded right the protein will not be able to do its job and it will attract the attention of proteosomes. Think of proteosomes as garbage collectors trolling the neighborhood looking for trash to pickup and dispose. Improperly folded proteins are the red flag for proteosomes. The offending mess is broken up into pieces and carted away for disposal - and most often the cell that contained the mis-folded protein is killed in the process. It turns out proteins cannot fold themselves, any more than your laundry can. They need external help to get the wrinkles out and get folded right. “Heat shock proteins” are the housekeepers (also called “chaperones”) that do the job. Hsp90 is the particular heat shock protein that is involved in the correct folding of ZAP-70. Now you get the picture: if we make sure the chaperone (Hsp90) is out to lunch (blocked by 17-AAG), the laundry (ZAP-70) does not get folded right, garbage men (proteosomes) assume it is garbage and destroy the crumpled stuff (misfolded ZAP-70). As we said above, ZAP-70 is an important part of the B-cell signaling mechanism. Get the ZAP-70 out of the way, the cell cannot proliferate and may soon die.
This is exciting and elegant science. You might well ask, are there other cells (besides B-cells) that express ZAP-70? Perfectly innocent cells that we do not want to get killed, cells that we need for our continued good health? The answer is yes. Perfectly healthy and normal T-cells should and do express ZAP-70 - in fact that is one of the reasons why it is so complicated to do the ZAP-70 measurement right. T-cells get in the way. Does this mean T-cells are also killed in the process of blocking Hsp90? That would be too bad, since destruction of B-cells and T-cells in one fell swoop sets patients up for too much immune suppression, a window of vulnerability when opportunistic infections and secondary cancers can strike.
Blood First Edition Paper, prepublished online June 21, 2026; DOI 10.1182/blood-2005-03-1099.
ZAP-70 is a novel conditional heat shock protein 90 (Hsp90)-client protein: inhibition of Hsp90 leads to ZAP-70 degradation, apoptosis and impaired signaling in chronic lymphocytic leukemia
Januario E Castro*, Carlos E Prada, Olivier Loria, Adeela Kamal, Liguang Chen, Francis J Burrows, and Thomas J Kipps
Moores UCSD Cancer Center, University of California, San Diego, CA, USA; Chronic Lymphocytic Leukemia Research Consortium (CRC), San Diego, CA.
Conforma Therapeutics Corporation, San Diego, CA.
ZAP-70 is expressed in patients with aggressive chronic lymphocytic leukemia (CLL). We found that ZAP-70+ CLL cells, expressed activated heat-shock protein 90 (Hsp90) with high binding-affinity for Hsp90-inhibitors, such as 17-allyl-amino-demethoxy-geldanamycin (17-AAG), whereas normal lymphocytes or ZAP-70-negative CLL cells expressed non-activated Hsp90. Activated Hsp90 bound and stabilized ZAP-70, which behaved like an Hsp90 client protein only in CLL cells. Treatment with Hsp90 inhibitors such as 17-AAG and 17-(dimethylaminoethylamino)-17-demethoxygeldanamycin (17-DMAG), induced ZAP-70 degradation and apoptosis in CLL cells but not in T cells, and also impaired B-cell receptor signaling in leukemia cells. Transduction of ZAP-70-negative CLL cells with an adenovirus encoding ZAP-70 activated Hsp90 and specifically rendered the leukemia cells sensitive to 17-AAG. These data indicate that Hsp90 is necessary for ZAP-70 expression and activity; that ZAP-70 is unique among Hsp90 clients, in that its chaperone-dependency is conditional upon the cell type in which it is expressed, and also that ZAP-70 is required for cell survival and signaling in CLL. Additionally, ZAP-70 expression in CLL cells confers markedly heightened sensitivity to 17-AAG or 17-DMAG, suggesting that these or other Hsp90 inhibitors could be valuable therapeutically in patients with aggressive CLL.
According to this paper, it seems the services of an activated form of Hsp90 is needed for the correct folding of ZAP-70 in CLL cells. It is this activated form of Hsp-90 that is targeted and blocked by the new drug 17-AAG. In other words, there is reason to hope that 17-AAG is a lot more toxic to ZAP-70 positive CLL cells, less toxic to regular B-cells or even CLL cells that do not express ZAP-70. T-cells are spared as well. This gives us the much desired highly targeted bang for the buck: poor prognosis ZAP-70 positive CLL cells are killed in preference to normal B-cells or T-cells.
But it may well turn out that 17-AAG is not for every CLL patient. If you are one of the lucky ones that has good prognostics and a slowly progressing variety of CLL that does not express ZAP-70, you may not respond to this drug. But then, you probably don’t care about that, you would be too busy getting on with your life - playing golf or whatever - and not thinking about CLL all that much anyway.
 |
HSP-90 |
The research presented in the Blood paper above was conducted with cell lines, and there are several bridges to cross before it becomes a done deal in real live patients. But as far as this research goes, the news is quite encouraging. There is interesting science, a new approach to things and 17-AAG may offer a brand new option for poor prognosis patients. It is also encouraging that a Phase-I clinical trial has kicked off, and that it is available at a bunch of centers across the country. I can smell excitement in the air, let us keep our fingers crossed. This is a small drug molecule. Dare we hope this slender and svelte molecule can get into all the nooks and crannies of lymph nodes and bone marrow, places where “fat lady” monoclonals like Rituxan and Campath cannot reach? That is an important question.
But, as always, the devil is in the details. I urge you to read the full text version of the abstract above, as well as our earlier article on 17-AAG to get some of the background information. Two of the authors on the Blood paper work for Conforma Therapeutics Corporation. The website for this company now leads to the Biogen Idec corporate website — where mention of 17AAG is hard to find. However, there is considerable molecular data on this and related compounds on a website run by a company called Invivogen: Geldanamycin and Analogues. Go figure. You need a scorecard to keep track of these players.
Below is some nuts-and-bolts information about the clinical trial I pulled together. You can also get a lot more details from the clinicaltrials.gov link: NCT00098488.
This Phase-I clinical trial is designed to get a handle on potential side effects as well as decide the best / safest dose of 17-AAG (“Dose Escalation Study”) and also see if there is synergy with the familiar (Rituxan + fludarabine “RF”) combination.
Patients who volunteer for this clinical trial will get 17-AAG (intravenous infusion, should take about 1 hour) on days 1, 4, 8, 11, 15, and 18. These six infusions will be repeated every 4 weeks, for a total of 6 cycles, provided there are no heavy duty adverse effects or disease progression. 17-AAG dosage will be gradually increased in different sets of patients, in order to nail down the “Maximum Tolerated Dose”, the level at which your body is ready to say “uncle”.
For patients who fail to get at least a 25% reduction in absolute lymphocyte counts after the first cycle, or where the ALC numbers get worse after any cycle, the researchers will get them a little extra help by adding fludarabine and Rituxan on top of the 17-AAG. For these patients, intravenous fludarabine will be given days 1-5 and Rituxan on day 1 of every course. To get things off to a fast start, Rituxan will be given on days 1, 3 and 5 of the first cycle.
Much though we like to be optimistic, new agents and new protocols carry a certain amount of risk. Taking a chemo-naïve patient, or one with good prognostics and sticking them into a clinical trial with potential for undue risk is just too darn aggressive for my taste. “Do no harm” is an important concept. We are fortunate that today newly diagnosed patients, especially if they have good prognostics, have a whole host of very attractive options. Why risk these people with an unproven therapy? Yet, we need clinical trials if we are to develop new and better options down the road. But we need to be careful not to expose volunteers to unreasonable risk.
This clinical trial has the right attitude. It is geared for fludarabine refractory patients. These are the classic “tough-nut to crack” cases, and prior to the advent of Campath and the like there were few good options left for patients who have already flunked fludarabine. Bone marrow transplants are not for everyone, for a variety of reasons. In other words, by limiting the recruitment to patients who have been around the block a few times, no low impact options left, and in need of therapy because their disease is on the war path, this clinical trial offers a very good option. Sure, there is risk. As I keep repeating, there is no free lunch in cancer therapy. But this trial offers hope where there may be few bright options left.
In fact, Combination immuno-chemotherapy such as Rituxan + fludarabine (“RF”), FRC (throw in cyclophosphamide as well, why not), and FluCam (fludarabine + Campath) are among the popular choices for refractory patients. I like the design of this clinical trial, in the sense that if the new drug 17-AAG does not seem to be working too well for the patient, “RF” is thrown in for good measure. Two for the price of one, as it were.
Basically, if you have advanced disease, failed fludarabine, spleen / lymph nodes etc are swollen and things are not looking so good and staying in Watch & Wait is no longer an option, you may be eligible. Click on the link we gave above to read the fine print. Oh yes, before I forget, you are not eligible if you are pregnant, kid under 18, presently indulging in other therapies, likely to drop dead in the very near future, or if you are nuts.
This clinical trial is offered at a number of the CLL Research Consortium sites. The protocol chair is Dr. Thomas Lin, member of Dr. John Byrd’s CLL team. Dr. Lin’s phone number is given below. I understand it is open for business at Ohio State, soon to be followed by Dana Farber. Other CRC sites may follow in the future. This trial is currently recruiting patients. If you think you might qualify and you are interested, there are two ways to go: you can print out this information and get your local oncologist to call on your behalf, or you can make the call yourself.
Dr. Thomas Lin
Ohio State University
Columbus, OH
Ph: 614-293-3507; 800-293-5066
There has been hype about miracle drugs before, and we can all remember being disappointed when some of these drugs did not make it past early phase trials. 17-AAG is a new drug, we could easily have a case of deja-vu all over again. Quoting from the same high-brow philosopher, the fat lady has just begun to hum. The tune sounds good, we hope we will like the song she is about to sing, and keep our fingers crossed there will be no false notes in her song. If your local oncologist is busy, shy, or plain not interested, there is absolutely no reason why you cannot make the call yourself to find out the details of this clinical trial. May be you are eligible, and with more information in your hand you can convince your local guy to go along with it as well. If you do get to participate in the trial, don’t forget to drop us a line, tell us how you are doing, OK? As always, we will be very careful not to expose your identity; but every little bit of information helps the patient community as a whole.
 Enter Keywords: |
———
Disclaimer: The content of this website is intended for information only and is NOT meant to be medical advice. Please be sure to consult and follow the advice of your doctors on all medical matters.
Copyright Notice:
Copyright © 2026-2007 CLL Topics, Inc. All Rights Reserved.
All materials contained on this site are protected by United States copyright law and may not be reproduced, distributed, transmitted, displayed, published or broadcast without the prior written permission of CLL Topics, Inc. You may not alter or remove any trademark, copyright or other notice from copies of the content.
However, you may download and print material from CLLTopics.org exclusively for your personal, noncommercial use.
———
