 |
||||||||||
Updated: February 9, 2026
Single Agent Fludarabine: Definitely Not the Gold Standard
UK CLL4 Clinical Trial Results
AIHA: One More Reason to Shun Single Agent Fludarabine
 Single agent fludarabine and single agent chlorambucil have often been the conventional choice for first line treatment of CLL. The use of these single agent therapies has continued even while there was ample evidence that combination therapies produced more impressive response statistics. Now we have results from the UK CLL4 clinical trial, a head-to-head comparison of single agent fludarabine, single agent chlorambucil and a combination of fludarabine and cyclophosphamide. In this article, Single Agent Fludarabine: Definitely Not the Gold Standard, we examine the role of fludarabine monotherapy in precipitating potentially life threatening AIHA. (2/09/08)
Single agent fludarabine and single agent chlorambucil have often been the conventional choice for first line treatment of CLL. The use of these single agent therapies has continued even while there was ample evidence that combination therapies produced more impressive response statistics. Now we have results from the UK CLL4 clinical trial, a head-to-head comparison of single agent fludarabine, single agent chlorambucil and a combination of fludarabine and cyclophosphamide. In this article, Single Agent Fludarabine: Definitely Not the Gold Standard, we examine the role of fludarabine monotherapy in precipitating potentially life threatening AIHA. (2/09/08)
Treanda: A Facelift for Bendamustine
Old Drug Recycled as New Therapy
Cephalon Makes a Splash with Orphan Drug for CLL
 Recent presentations at the ASH 2026 meeting and a flurry of press releases on Treanda (bendamustine hydrochloride) have raised a considerable level of interest in the drug. While the agent itself has been around for over thirty years, its development as a drug for use in CLL is more recent. In our article, Treanda: A Facelift for Bendamustine, we examine the results of clinical trials reported to date and offer our analysis of the clinical trial results and the potential value of this drug in treating CLL. (12/15/07)
Recent presentations at the ASH 2026 meeting and a flurry of press releases on Treanda (bendamustine hydrochloride) have raised a considerable level of interest in the drug. While the agent itself has been around for over thirty years, its development as a drug for use in CLL is more recent. In our article, Treanda: A Facelift for Bendamustine, we examine the results of clinical trials reported to date and offer our analysis of the clinical trial results and the potential value of this drug in treating CLL. (12/15/07)
Refractory CLL
Celgene Targets Revlimid as CLL Therapy
Revlimid to the Rescue?
 Oncology drug maker, Celgene Corp., sponsored two recent phase II clinical trials for the use of its drug Revlimid, in CLL. The results of these trials were reported in the 2026 ASH annual meeting in Orlando. In our article, Revlimid to the Rescue?, we review the results of these trials and present our commentary on the drug's efficacy, adverse effects and potential value in cases with difficult prognostic factors. Any CLL patient who is asked to consider off label use of this drug or to participate in Celgene's big follow-up phase III clinical trial needs to do his or her homework first — starting by reading this article. (1/30/07)
Oncology drug maker, Celgene Corp., sponsored two recent phase II clinical trials for the use of its drug Revlimid, in CLL. The results of these trials were reported in the 2026 ASH annual meeting in Orlando. In our article, Revlimid to the Rescue?, we review the results of these trials and present our commentary on the drug's efficacy, adverse effects and potential value in cases with difficult prognostic factors. Any CLL patient who is asked to consider off label use of this drug or to participate in Celgene's big follow-up phase III clinical trial needs to do his or her homework first — starting by reading this article. (1/30/07)
Multi-center Controlled Results
Is More Necessarily Better?
A Direct Comparison of FC vs F
 The latest issue of Blood carries an article from the German CLL Study Group
comparing single-agent fludarabine against a combination of fludarabine plus
cyclophosphamide. This is a well-structured, rigorous study and the study group
comes to some surprising conclusions. Read our analysis, F+C vs F, of the study and comments
from an editorial in Blood by Dr. Neil Kay. (3/18/06)
The latest issue of Blood carries an article from the German CLL Study Group
comparing single-agent fludarabine against a combination of fludarabine plus
cyclophosphamide. This is a well-structured, rigorous study and the study group
comes to some surprising conclusions. Read our analysis, F+C vs F, of the study and comments
from an editorial in Blood by Dr. Neil Kay. (3/18/06)
Novel Agents
Hope Springs Eternal
Latest on Candidate 17-AAG
 The patient community is ever hopeful about new drug candidates that appear to have potential in the lab. In 17-AAG we examine a new agent that is receiving attention, 17-AAG, which is on its way to starring in a clinical trial at Ohio State under the orchestration of Dr. John Byrd. (6/20/05)
The patient community is ever hopeful about new drug candidates that appear to have potential in the lab. In 17-AAG we examine a new agent that is receiving attention, 17-AAG, which is on its way to starring in a clinical trial at Ohio State under the orchestration of Dr. John Byrd. (6/20/05)
Target Mitochondrion
Promising New Approaches Bypass the Usual Cellular Control Points and May Level the Playing Field for Bucket C Patients
New Research on Attacking Mitochondria in B-CLL Cells
 Several recent research articles have opened up exciting new opportunities in the treatment of CLL. These feature new small molecule drugs that take an entirely different approach to targeting CLL cells. In this new approach to treating CLL, it seems to make no difference if you are IgVH mutated or unmutated, CD38 positive or not, chemo-naïve or have been through the wars with every chemotherapy drug known to man. In fact, there are some indications that heavily pretreated and late Rai stage patients may respond better to this approach. To learn more about these exciting new drugs on the horizon, read Target Mitochondrion. (9/24/04)
Several recent research articles have opened up exciting new opportunities in the treatment of CLL. These feature new small molecule drugs that take an entirely different approach to targeting CLL cells. In this new approach to treating CLL, it seems to make no difference if you are IgVH mutated or unmutated, CD38 positive or not, chemo-naïve or have been through the wars with every chemotherapy drug known to man. In fact, there are some indications that heavily pretreated and late Rai stage patients may respond better to this approach. To learn more about these exciting new drugs on the horizon, read Target Mitochondrion. (9/24/04)
Flavopiridol
NCI Develops Experimental Drug
A Drug that May Save Lives
 We review preclinical data and early results from clinical trials with flavopiridol, an important experimental drug. Flavopiridol is being developed by Aventis Oncology in collaboration with the National Cancer Institute. Its use is presently under investigation for a variety of solid tumors as well as hematological cancers. It offers the possibility of p53- and ATM-independent cell kill in CLL, thereby opening an important therapeutic path for patients with poor-prognosis cytogenetics. While there are issues related to the drug's toxicity, there is also hope that it could survive the early phase optimization trials to become a major drug for the treatment of CLL. Read about the potential value and possible drawbacks of this agent in Flavopiridol: A Drug that May Save Lives. (6/6/04)
We review preclinical data and early results from clinical trials with flavopiridol, an important experimental drug. Flavopiridol is being developed by Aventis Oncology in collaboration with the National Cancer Institute. Its use is presently under investigation for a variety of solid tumors as well as hematological cancers. It offers the possibility of p53- and ATM-independent cell kill in CLL, thereby opening an important therapeutic path for patients with poor-prognosis cytogenetics. While there are issues related to the drug's toxicity, there is also hope that it could survive the early phase optimization trials to become a major drug for the treatment of CLL. Read about the potential value and possible drawbacks of this agent in Flavopiridol: A Drug that May Save Lives. (6/6/04)
Insight
The "How" Is Often as Important as the "What" in Chemotherapy
Methods of Drug Delivery
 This article offers important insights into the effectiveness of various methods of drug delivery. The results you get may be vastly different depending upon the precise dosing, timing and method of delivery involved in the protocol you are following. Sometimes the results are counter-intuitive. To learn about the effect of bioavailability and pharmacokinetics on the efficacy of therapies, read Chemotherapy: Methods of Drug Delivery. (10/20/03)
This article offers important insights into the effectiveness of various methods of drug delivery. The results you get may be vastly different depending upon the precise dosing, timing and method of delivery involved in the protocol you are following. Sometimes the results are counter-intuitive. To learn about the effect of bioavailability and pharmacokinetics on the efficacy of therapies, read Chemotherapy: Methods of Drug Delivery. (10/20/03)
Dosage Is Critical
Recipies Vary Widely
Drug Dosages in Popular “Standard” Protocols
 As results are published from the various chemoimmunotherapy clinical trials, the protocols themselves can be compared. Drug dosage, timing and method of delivery can all have a major impact on the efficacy, toxicity and mutagenicity of a given protocol. In our article, Drug Dosages in Popular “Standard” Protocols, we compare the dosage of the critical active elements across a number of popular protocols — and add our two cents of editorial commentary. (9/13/03)
As results are published from the various chemoimmunotherapy clinical trials, the protocols themselves can be compared. Drug dosage, timing and method of delivery can all have a major impact on the efficacy, toxicity and mutagenicity of a given protocol. In our article, Drug Dosages in Popular “Standard” Protocols, we compare the dosage of the critical active elements across a number of popular protocols — and add our two cents of editorial commentary. (9/13/03)
Failures in Therapy
Things that Somehow Didn't Work Out
Overcoming the Spin with Facts
 There are many reasons why promising therapy approaches often do not work out in practice, some to do
with the basic biochemistry of the disease and the immune system and some to do with economics or the
vagaries of our healthcare system. Often the therapy under investigation has deficiencies that can only
be revealed by a clinical trial but often issues are identifiable even before a trial is launched. Read
our discussion of these matters in
Failures in Therapy. (8/30/03)
There are many reasons why promising therapy approaches often do not work out in practice, some to do
with the basic biochemistry of the disease and the immune system and some to do with economics or the
vagaries of our healthcare system. Often the therapy under investigation has deficiencies that can only
be revealed by a clinical trial but often issues are identifiable even before a trial is launched. Read
our discussion of these matters in
Failures in Therapy. (8/30/03)
NO-NSAIDs
A New Class of Agents May Prove Useful in Cancer Control
Some Early Results
 A new class of NSAIDs called NO-NSAIDs has been developed: these moleculess offer the prospect
of more effective treatment of inflammation without some of the damaging side effects of high dose
traditional NSAIDs. They also show indications in the lab that they are effective in cancer cell
kill and possibly, in-vivo tumor control. A number of these drugs are now in development and you may
soon be hearing about them in clinical trials. To learn more, read our article on
NO-NSAIDs. (8/30/03)
A new class of NSAIDs called NO-NSAIDs has been developed: these moleculess offer the prospect
of more effective treatment of inflammation without some of the damaging side effects of high dose
traditional NSAIDs. They also show indications in the lab that they are effective in cancer cell
kill and possibly, in-vivo tumor control. A number of these drugs are now in development and you may
soon be hearing about them in clinical trials. To learn more, read our article on
NO-NSAIDs. (8/30/03)
Multi Drug Resistance
Cancer Cells Subvert the Body's Defence Secrets for Their Own Purposes
A Significant – and Inevitable – Obstacle
 Chemotherapy has its limitations. In CLL, the tumor always seems to find ways of resisting the drugs used, so that chemotherapy protocols become less effective over time and ultimately fail. When the cancer cells learn the trick of drug resistance, they often find ways to become resistant to a whole range of drugs — hence the term Multiple Drug Resistance. To learn more, read our article bearing the title: Multi Drug Resistance to Chemotherapy. (7/23/03)
Chemotherapy has its limitations. In CLL, the tumor always seems to find ways of resisting the drugs used, so that chemotherapy protocols become less effective over time and ultimately fail. When the cancer cells learn the trick of drug resistance, they often find ways to become resistant to a whole range of drugs — hence the term Multiple Drug Resistance. To learn more, read our article bearing the title: Multi Drug Resistance to Chemotherapy. (7/23/03)
Fludarabine
The Most Active Single Chemotherapy Agent in CLL ... So Far
No Longer the Gold Standard but Still in the Game
 Fludarabine, or its branded form, Fludara, has been a major component of most chemotherapy and
chemoimmunotherapy combinations specifically designed for CLL. Due to its maturity in the market,
there is a large body of information and experience available for this compound. Best practices
and side-effects are perhaps better understood than with other potent agents: and patients
should spend the time to understand what they are letting themselves in for before they sign
up for a fludarabine-containing protocol. The compound is now off-patent — which could
be a consideration if insurance limitations are a concern. To learn more, read
the articles found in our index page on Fludarabine. (Multiple dates.)
Fludarabine, or its branded form, Fludara, has been a major component of most chemotherapy and
chemoimmunotherapy combinations specifically designed for CLL. Due to its maturity in the market,
there is a large body of information and experience available for this compound. Best practices
and side-effects are perhaps better understood than with other potent agents: and patients
should spend the time to understand what they are letting themselves in for before they sign
up for a fludarabine-containing protocol. The compound is now off-patent — which could
be a consideration if insurance limitations are a concern. To learn more, read
the articles found in our index page on Fludarabine. (Multiple dates.)
The following are short earlier articles on chemotherapy, presented most recent first.
Therapy Approach
TRAIL — What Is It and How Does It Work?
Date: 6/1/03
by Chaya Venkat
One of our members expressed interest in TRAIL, here is what I have gathered thus far. Generally speaking, I think there are far too many acronyms, medical jargon to confuse the lay people like us. But here is one acronym that makes sense: "TRAIL" stands for "tumor necrosis factor-related apoptosis-inducing ligand". See what I mean?
A little bit of background, before we discuss TRAIL and what it might mean to us down the road. In the natural order of things, as cells get old and decrepit, or have just plain outlived their usefulness, they are programmed to commit suicide when commanded to do so by the body sending them appropriate signals. This orderly process of cellular death is called "apoptosis". All hell breaks loose if the cells stop obeying the call to kill themselves, because that means useless and unwanted cells gradually accumulate in large numbers, eventually grinding the efficient running of the body to a halt. This is one aspect of cancer, the most important aspect of it from the perspective of CLL.
"Tumor Necrosis Factor" or TNF is one of the death signals the body uses, and other members of the family includes Fas-ligand (FasL) and TRAIL. When a cell is targeted for death, members of the TNF family attach themselves to the appropriate docking stations ("receptors") on that cell, and the job gets done. We are now able to make many of these biologically active compounds in the lab. TNF-alpha was the first molecule to be tested for its anti-tumor activity, followed by Fas-ligand. These two molecules are efficient in killing a variety of tumor cells. However, they cause significant damage to normal tissues that result in life-threatening toxicities. A case of patient getting cured of cancer, but unfortunately dying in the process. Therefore, the search continued until the recently discovered new member of the TNF family, TRAIL.
TRAIL has been shown to be selectively toxic, causing suicide of tumor cells and with minimal no toxicity against normal tissues, as demonstrated in cell studies, and animal studies using mice and monkeys. TRAIL is expressed in many human tissues, (although not in the liver and brain), which suggests that TRAIL does not have a toxic effect on normal cells. In fact, many normal primary cells such as epithelial cells, fibro-blasts, and skeletal muscle cells are resistant to TRAIL-induced apoptosis.
TRAIL has two docking stations on cells, called death-receptors (DR4 and DR5). In addition, there are two 'decoy' receptors for TRAIL, called DcR1 and DcR2, which are there to fake out the TRAIL molecule, prevent it from killing normal cells. If TRAIL is to be a successful drug for selectively killing cancer cells, the hope is that these malignant cells have lots and lots of the correct death-receptors, DR4 and DR5, and not the decoy receptors DcR1 and DcR2.
Recently, there is controversy that while most normal cells are not killed by TRAIL, liver cells could be an exception. This was not observed when TRAIL was tested with mice and monkeys, but here is a case where humans may be different from mice and monkeys. The first PubMed citation below draws a pretty blunt conclusion, "substantial liver toxicity might result if TRAIL were used in human cancer therapy". Just to keep us plain folk guessing, the second PubMed citation disagrees completely with the first one: these authors think TRAIL does not kill normal liver cells, only those that are already infected with hepatitis virus. Good thing too, these authors think, in fact TRAIL should be used not only for cancer, but also for treating hepatitis!! Go figure.
TRAIL is expected to promotes apoptosis in cancer cells harboring death receptors, even in the presence of "oncogenes" Bcl-2 and Bcl-XL, which would otherwise protect the cancer cells from dying. In addition, in cell studies and mouse studies, TRAIL boosts the killing effects of chemotherapy drugs or radiation; therefore, the dose of chemotherapy drugs or radiation can be reduced, if they are combined with TRAIL in therapy protocols. The bottom line to the good news is that whereas most chemotherapy drugs and radiation are toxic to good cells as well as cancer cells, TRAIL kills cancer cells effectively, but it is non-toxic (barring the controversy about liver damage discussed above).
If in one of your generous moments you might have sent money off to the Leukemia & Lymphoma Society, you might like to know part of your money went to support TRAIL research of two Canadian scientists. The press release is below, dated 2026. I tried to see if there were any updates from their research, but I was not able to locate any. Please write if you have additional information on this score. Two years is a long time in today's break-neck speed of biotechnology development.
The only PubMed citation that I have found that directly connects TRAIL therapy in CLL is disappointing. It appears that B-cells from CLL are particularly resistant to TRAIL, because CLL cells express on their surface only low levels of the correct death-inducing TRAIL receptors, and high levels of the 'decoy' receptors. Same sort of a reason why Rituxan works better in follicular lymphoma than in CLL, because there are more CD20 markers for the Rituxan to latch on to in follicular lymphoma than there are in CLL. Scientists are now working to see how they can up-regulate the expression of the right death receptors in CLL cells, possibly by co-administering TRAIL along with more familiar chemotherapy drugs such as Cyclophosphamide. Somehow, that makes its appeal a lot less sexy to me.
I have looked but found no references, yet, to clinical trials using TRAIL for CLL, or even for our kissing cousin NHL. While this technology is certainly interesting and worth watching, I do not expect there will be 'home runs' any time soon. These days, my definition of "soon" is determined by the median life expectancy of CLL patients. And the caveats I have seen thus far, especially the reference to the particular resistance of CLL cells to this drug, makes me pragmatic about its possible use for us. If I were to guess, my optimistic bet would be that TRAIL would become another very valuable drug in our fight against CLL, one that will have particularly good response in some CLL patients, but not all of us. Its best use is likely to be in combination with other chemotherapy drugs, and as salvage therapy for patients who are refractory to more conventional chemotherapy. In that sense, if the research pans out, TRAIL is likely to take its place alongside of Rituxan and Campath, in 5-10 year time frame.
Nat Med 2026 May;6(5):564-7
Apoptosis induced in normal human hepatocytes by tumor necrosis factor-related apoptosis-inducing ligand.
Jo M, Kim TH, Seol DW, Esplen JE, Dorko K, Billiar TR, Strom SC.
Department of Pathology, School of Medicine, 200 Lothrop St. BST S-450, University of Pittsburgh, Pittsburgh, PA
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) has been reported to induce apoptosis in various tumor cells but not in nontransformed, normal cells. Preclinical studies in mice and nonhuman primates have shown that administration of TRAIL can induce apoptosis in human tumors, but that no cytotoxicity to normal organs or tissues is found. The susceptibility of tumor cells to TRAIL and an apparent lack of activity in normal cells has lead to a proposal to use TRAIL in cancer therapy. Here, we assessed the sensitivity of hepatocytes from rat, mouse, rhesus monkey and human livers to TRAIL-induced apoptosis. TRAIL induced apoptosis in normal human hepatocytes in culture but not in hepatocytes isolated from the other species. Human hepatocytes showed characteristic features of apoptosis, including cytoplasmic shrinkage, the activation of caspases and DNA fragmentation. Apoptosis and cell death in human hepatocytes was massive and rapid, occurring in more than 60% of the cells exposed to TRAIL within 10 hours. These results indicate that there are species differences in sensitivity to TRAIL, and that substantial liver toxicity might result if TRAIL were used in human cancer therapy.
PMID: 10802713
____________
FASEB J 2026 Jan;17(1):94-6
Involvement of TRAIL and its receptors in viral hepatitis.
Mundt B, Kuhnel F, Zender L, Paul Y, Tillmann H, Trautwein C, Manns MP, Kubicka S.
Department of Gastroenterology and Hepatology, Medizinische Hochschule Hannover, Germany.
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is able to kill a broad spectrum of tumor cells but appears to be nontoxic to most normal cells. Because there are conflicting data about the hepatotoxicity of TRAIL, we investigated the physiological function of TRAIL and its receptors in the liver. Hepatocytes are sensitive for FasL- and TRAIL-mediated apoptosis in vitro, but TRAIL induces no apoptosis in healthy livers in vivo. Using mouse models of adenoviral hepatitis and livers of patients with hepatitis infection, we could demonstrate that apoptosis in virally infected hepatocytes is mediated by TRAIL receptor DR5 and TRAIL. In contrast to FasL, TRAIL-mediated apoptosis of hepatocytes in vivo is triggered through viral infection. The TRAIL receptor/ligand system enables the organisms to specifically kill virus-infected hepatocytes, whereas normal uninfected hepatocytes in vivo are resistant to TRAIL-mediated apoptosis. Overexpression of TRAIL in the liver after viral infection is not dependent on lymphocytes, natural killer, or Kupffer cells, which indicates that the TRAIL receptor/ligand system is a paracrine mechanism of hepatocytes against virally infected cells. Our results suggest that TRAIL might be used not only for cancer therapy but also for therapy of patients with viral hepatitis to selectively eliminate infected hepatocytes and limit viral replication.
PMID: 12475902
____________
Sept 28, 2026
Manitobans receive prestigious international award
Research may lead to new treatment, eventual cure for adult leukemia
(Winnipeg) Two researchers from the Manitoba Institute of Cell Biology (MICB) at CancerCare Manitoba received a prestigious international award this month to further research that may lead to new treatments and the possible eventual cure for what can be a terminal form of cancer.
Chronic Lymphocytic Leukemia (CLL) is an incurable leukemia that mainly affects older adults. Therapy is designed to lengthen and improve the quality of life for patients and is required in about half of all cases.
Dr. Spencer Gibson and Dr. James Johnston received a prestigious award from an American non-profit organization, the Leukemia & Lymphoma Society, to study the role of a naturally occurring protein, TRAIL in treating CLL. TRAIL effectively kills cancer cells while leaving normal cells alone. Initial findings by these researchers in the laboratory indicated the patient’s CLL cells die following treatments with TRAIL while normal blood cells remain healthy.
“The Society’s Translational Research Program provides support for new and novel research that takes the discoveries of the laboratory bench and translates them into new treatments for blood-related cancers,” explains Alan Kinniburgh, Ph.D., vice president for research at The Leukemia & Lymphoma Society.
“Dr. Gibson and Dr. Johnston are our first Manitoba-based researchers. We're thrilled to welcome them to our worldwide team of researchers who are focused on finding clinical applications for their work, which will hopefully lead to cures for these devastating blood-related cancers.”
“This is a prime example of basic research being translated into the development of new treatments for cancer right here in Manitoba,” said Dr. Spencer Gibson, researcher. “It also demonstrates the enhanced insight gained when researchers work side-by-side with clinicians.”
MICB will receive $100,000 U.S. annually for the next three years to help fund the research. MICB is dedicated to fundamental research in biology and its relation to health, with a primary emphasis on cancer and related diseases. The MICB was founded jointly by CancerCare Manitoba and the University of Manitoba in 1969.
_____________
Oncogene 2026 Oct 3;21(44):6809-18
Mechanisms of resistance to TRAIL-induced apoptosis in primary B cell chronic lymphocytic leukaemia.
MacFarlane M, Harper N, Snowden RT, Dyer MJ, Barnett GA, Pringle JH, Cohen GM.
MRC Toxicology Unit, Hodgkin Building, University of Leicester, PO Box 138, Lancaster Road, Leicester LE1 9HN, UK.
Primary B cells from B cell chronic lymphocytic leukaemia (B-CLL) were resistant to the novel selective cytotoxic agent, TNF-related apoptosis-inducing ligand (TRAIL). Low levels of the death-inducing TRAIL receptors, TRAIL-R1 and TRAIL-R2 but not the putative 'decoy' receptors, TRAIL-R3 and TRAIL-R4, were expressed on the surface of B-CLL cells. Resistance to TRAIL was upstream of caspase-8 activation, as little or no caspase-8 was processed in TRAIL-treated B-CLL cells. Low levels of a TRAIL death-inducing signalling complex (DISC) were formed in these cells, accompanied by the recruitment of endogenous FADD, caspase-8 and c-FLIP(L) but not c-FLIP(S). Both caspase-8 and c-FLIP(L) were cleaved to form two stable intermediates of approximately 43 kDa, which remained associated with the DISC. Caspase-8 was not further processed to its active heterotetramer. Thus the resistance of B-CLL cells to TRAIL may be due partly to low surface expression of the death receptors resulting in low levels of DISC formation and also to the high ratio of c-FLIP(L) to caspase-8 within the DISC, which would prevent further activation of caspase-8. Our results highlight the possibility of sensitising B-CLL cells to TRAIL by modulation of c-FLIP levels or by upregulation of surface expression of death receptors.
PMID: 12360407
_____________
(Chaya's note: these authors use a slightly different naming system: They call the death receptors DR4 and DR5 as R1 and R2, and the two 'decoy' receptors DcR1 and DcR2 are called R3 and R4. If all these guys were on the same page, used the same names for stuff, it would make our job of trying to figure these things out a little easier).

Protection Against Bone Loss Resulting from Steroid Therapy
Date: 5/20/03
by Chaya Venkat
Prednisone and other glucosteroids are ever popular in treatment of CLL. Earlier today I wrote on the risk of potential congestive heart failure associated with use of Hydroxydoxorubicin (or doxorubicin for short), the "H" in the "CHOP" chemotherapy combination used frequently to treat CLL, PLL and RS. Now it is the turn of Prednisone, the "P" in CHOP.
Glucosteroids like prednisone (or methylprednisolone) are effective immune system suppressors. As such, they are frequently used in treating chronic autoimmune problems like rheumatoid arthritis etc. Their ability to kill immune system cells also makes them useful in treating diseases like CLL, where we have a super-abundance of B-cells. Their immune suppressive effects can also help with anemia and thrombocytopenia associated with autoimmune destruction of red blood cells and platelets in CLL patients. One of our members has undergone low dose prednisone therapy to first take care of low RBC due to AIHA (autoimmune hemolytic anemia) when he tested positive for the Coomb's test, followed by Rituxan therapy. The patient has had excellent results with this combination. I personally think he, his wife and their ever so prudent and careful oncologist deserve a hand for this excellent response.
My article today has to do more with high dose methylprednisolone (or other glucosteroids) in combination with Rituxan. This particular combination is in clinical trials at a number of CLL consortium centers. We have discussed it in other articles. See: Rituxan plus Prednisone, Prednisone, the Good, the Bad and the Downright Ugly, Rituxan plus HDMP Clinical Trial and Topics Alert # 57: R plus HDMP.
There is some logic for this combination, there are indications that the glucosteroid and Rituxan have synergistic effects: the former is thought to up-regulate CD20, and down-regulate complement inhibitory markers such as CD55 and CD59, all of which is good in terms of enhancing the effectiveness of Rituxan. However, there are serious and long term implications of high dose glucosteroidal use, one of which is significant loss of bone mass density. The risks of hip and spine fractures as a result of bone density losses following glucosteroidal use are quite real and could be serious long term health risks, especially for post-menopausal women. The risks are increased with increased use of glucosteroids, especially if the steroid therapy is stopped abruptly. It is important to wean off of the steroids gradually.
Since glucosteroids are a fact of life in CLL therapy for the near future, I thought you guys might be interested in learning about "Fosamax", a drug that is well tolerated and well researched to protect against steroid induced loss of bone density. If prednisone or its next of kin are in your future, you might want to save this information, it might make it easier to convince your oncologist to have this drug prescribed for you. If you type in Fosamax (or alendronate, the generic name) into PubMed, you get hundreds of hits. I picked one almost at random, and it is attached below. The NEJM article report below is in some ways easier to read and more informative for our purposes.
If you are going to indulge in high dose glucosteroids, remember the following points: (1) ask your oncologist about protection against osteoporosis, specifically about drugs such as Fosamax. (2) ask your oncologist if it is possible to taper off the glucosteroid use gradually, rather than cold turkey. Believe me, besides the osteoporosis risks, you will not enjoy the psychological roller-coaster ride of sudden glucosteroid withdrawal.
Another member had this important point to make regarding the effects of steroid therapy: "... another potentially serious side effect of high dose prednisone is induced diabetes. My endocrinologist has warned me about this and my onc has discussed it with her. Apparently, the prednisone can actually curtail islet cell production and increase insulin resistance, a very bad combination. Just something to check out with your MD before and during pred medication..."
University of Iowa College of Medicine
Effective Treatment for Steroid-Induced Osteoporosis
Description: University of Iowa researchers report in the July 30 issue of The New England Journal of Medicine that the drug alendronate (marketed as Fosamax) may help prevent and treat steroid-induced osteoporosis. 7/29/98
Health News 2130 Medical Laboratory
IOWA CITY, Iowa -- University of Iowa researchers, et al., report in the July 30 issue of The New England Journal of Medicine that the drug alendronate (marketed as Fosamax) may help prevent and treat steroid-induced osteoporosis.
UI researchers, led by Kenneth Saag, M.D., assistant professor of internal medicine, and investigators from 14 other U.S. and 22 international sites, detail the results of two 48-week studies of 477 men and women ages 17 to 83 receiving 7.5 mg or greater of prednisone (or equivalent) daily. The studies examined the effectiveness of alendronate in preventing and treating osteoporosis among patients undergoing steroid therapy.
"Steroids such as prednisone are often prescribed by doctors for a number of medical conditions, including rheumatoid arthritis, asthma and inflammatory bowel disease," Saag said. "While steroids are effective in treating these diseases, osteoporosis is often an unavoidable, yet serious, long-term side effect."
Patients in the studies received either an oral dose of alendronate (5 mg to 10 mg) or an inactive placebo. All the patients also were given calcium (800 mg to 1000 mg) and vitamin D supplements (250 to 500 IU), which are currently recommended for preventing and treating steroid-induced osteoporosis.
The researchers found that either dose of alendronate, added to calcium and vitamin D, significantly increased bone mineral density (BMD) -- the most important predictor of fracture risk -- at the spine and hip in men and women taking steroids compared with placebo (calcium and vitamin D). The results were consistent, regardless of the patient’s age, gender, underlying disease, dosage or length of time on steroid therapy.
Increase in spine BMD was highest in post-menopausal women not taking estrogen who received 10 mg of alendronate, the researchers noted. Post-menopausal women taking steroid treatments are among those at the highest risk for steroid-induced osteoporosis, due to the combined detrimental effects of estrogen deficiency and steroids on their bones.
The studies also showed fewer patients on alendronate had spine fractures compared with those patients on placebo.
Researchers already knew that alendronate could prevent and treat postmenopausal osteoporosis and prevent fractures, Saag noted, but the new study findings show that the drug can also play a role in preventing and treating osteoporosis caused by steroids.
Of the 30 million American men and women who have diseases that may require treatment with glucocorticoid steroids, an estimated one million people presently use them on a chronic basis.
"Early intervention is critical because steroid users lose large amounts of bone and lose it rapidly -- as much as 10 to 20 percent in the first year of steroid treatment," Saag said. "Approximately 50 percent of chronic steroid users develop osteoporosis, increasing their risk for fractures. Calcium and vitamin D supplements, hormone replacement therapy and exercise have been the recommended modes of therapy, but our studies show that alendronate provides additional benefit over and above calcium plus vitamin D."
In the studies, alendronate at 5 and 10 mg was generally well tolerated. Esophageal adverse experiences were not increased with alendronate treatment, nor were peptic ulcers despite concurrent use of steroids in all patients and extensive use of aspirin, non-steroidal anti-inflammatories and slow-acting anti-rheumatic drugs. Alendronate, marketed by Merck and Co., Inc., was first approved by the U.S. Food and Drug Administration in late 1995. It has been prescribed for approximately 2.4 million people in the United States for the treatment and prevention of post-menopausal osteoporosis and for the treatment of Paget’s disease of bone.
_______________
Arthritis Rheum 2026 Apr;48(4):1102-8 Related Articles, Links
Changes in bone mineral density following discontinuation or continuation of alendronate therapy in glucocorticoid-treated patients: a retrospective, observational study.
Emkey R, Delmas PD, Goemaere S, Liberman UA, Poubelle PE, Daifotis AG, Verbruggen N, Lombardi A, Czachur M.
Radiant Research, Wyomissing, Pennsylvania
OBJECTIVE: To evaluate the effects of discontinuing or continuing alendronate (ALN) therapy on bone mineral density (BMD) after patients on a long-term regimen of glucocorticoids (GCs) completed a 1-year treatment period with ALN.
METHODS: Eligible patients were individuals with GC-induced osteoporosis who had received ALN (5 or 10 mg) for 1 year in a prior clinical trial and, at the end of the year, were still taking GCs at an average daily dose of > or =7.5 mg of prednisone or equivalent. Patients were contacted 3-5 years after completion of the prior ALN trial for followup measurements of the lumbar spine BMD and hip BMD, and retrospective information was collected about serious or drug-related adverse experiences and concomitant medication use. Some patients remained on GCs, and some remained on ALN, either alone or in combination with other drugs. The primary response parameter was the percentage change in lumbar spine BMD from the end of year 1 to the followup visit. Change in BMD at the hip was a secondary response parameter.
RESULTS: Ninety (49.2%) of the eligible 183 patients participated in the retrospective study. The followup period, which began at the end of year 1 of the original clinical trial, ranged from 3.3 years to 4.6 years. The mean number of days of treatment with ALN was 507. Fifty patients were included in the analysis because they had received supraphysiologic doses of GCs (doses above the lowest tertile of GC use for the study population; that is, higher than approximately 6 mg/day), and they had not taken (defined as <6 months of use) other bone-affecting agents except ALN. Eleven of the 50 patients discontinued taking ALN (duration of use <90 days), 8 took ALN between 90 days and 300 days, and 31 continued to take ALN for >300 days after year 1 of the clinical trial. GC users who discontinued treatment with ALN (<90 days of therapy) had numerically greater decreases in BMD at the lumbar spine, femoral neck, and total hip from the end of year 1 (mean change -5.1%, -9.2%, and -6.6%, respectively), compared with patients who continued to take ALN for >300 days (mean change 0.1%, -0.9%, and 1.8%, respectively).
CONCLUSION: Substantial loss of BMD in the lumbar spine and hip was seen in patients who discontinued treatment with ALN but who continued to take >6 mg/day of GCs. However, patients receiving GCs who remained on the ALN regimen appeared to benefit from continued ALN treatment, since BMD was maintained in this latter group.
PMID: 12687554
____________

Doxorubicin - Risk of Heart Problems
Date: 5/20/03
by Chaya Venkat
 There are way too many acronyms in CLL, but some are more important than others. I am sure many of you have
heard of "CHOP" as a combination chemotherapy used for some patients. The "H" in CHOP
stands for hydroxydoxorubicin, also referred to as simply doxorubicin. There have always been concerns of
heart disease as one of the risk factors of doxorubicin administration. The breaking story below has been
carried by many news services, indicating that the risk of congestive heart failure is higher than previously
thought. If you are presently on this drug, or are likely to be on it in the near future, it pays to be a
little extra vigilant in having your heart functions monitored on a regular basis. Read the full article
which appeared in the Atlanta Journal-Constitution using the following URL. The introduction appears below:
There are way too many acronyms in CLL, but some are more important than others. I am sure many of you have
heard of "CHOP" as a combination chemotherapy used for some patients. The "H" in CHOP
stands for hydroxydoxorubicin, also referred to as simply doxorubicin. There have always been concerns of
heart disease as one of the risk factors of doxorubicin administration. The breaking story below has been
carried by many news services, indicating that the risk of congestive heart failure is higher than previously
thought. If you are presently on this drug, or are likely to be on it in the near future, it pays to be a
little extra vigilant in having your heart functions monitored on a regular basis. Read the full article
which appeared in the Atlanta Journal-Constitution using the following URL. The introduction appears below:
http://www.ajc.com/health/content/shared-auto/healthnews/hrts/513269.html;
Cancer Drug's Heart Risk Underestimated
MONDAY, May 19 (HealthScoutNews) -- Doctors have known a common cancer drug causes heart failure in some patients, forcing them to stop treatment and in rare cases requiring an organ transplant, but a new study says that risk may be significantly greater than previously believed. The drug, doxorubicin, is used in the treatment of many cancers, from leukemia and breast tumors to lung and testicular cancers. The new research doesn't second-guess the therapy. However, experts say it should prompt cancer specialists to be especially vigilant about budding congestive heart failure, particularly in patients most vulnerable to the problem, such as children, the elderly and those who've had radiation therapy...
For more on doxorubicin, visit the M.D. Anderson Cancer Center.

Pentostatin plus Cyclophosphamide
Date: 4/1/03
by Chaya Venkat
We have discussed Pentostatin before in prior articles, especially the clinical trial results of a study using Rituxan plus Pentostatin ("RP"), instead of the much more familiar Rituxan plus Fludarabine ("RF") combo. (See Pentostatin plus Rituxan Clinical Trial). Below is the second leg of the tripod, Pentostatin plus Cyclophosphamide. I understand Dr. Weiss of Memorial Sloan Kettering has a "RPC" (Rituxan, Pentostatin and Cyclophosphamide) trial underway, to compare against the better known "RFC" trials at Anderson and elsewhere.
Just to refresh your memory, Pentostatin is a purine analogue, just like its better known cousin Fludarabine. (Cladribine is the third member of this group of drugs). Pentostatin has proved to be an effective agent against Hairy Cell Leukemia (HCL), but it was considered to be not as effective in CLL. Nevertheless, there has been discussion that Pentostatin is less damaging to the bone-marrow than Fludarabine, and there should be comparisons of combo drug therapies using Pentostatin rather than Fludarabine.
23 previously treated CLL patients (some of them refractory to Fludarabine) were enrolled in this study. Pentostatin and Cyclophosphamide were administered over 6 sessions, over a period of 18 weeks. I was interested to see that G-CSF (protection against possible neutrophil deficit after therapy) and antiviral and antibacterial medications were routinely given to all patients. This is a good and prudent thing to do, I wonder why it is not done in all therapy regimes where there is a risk of neutropenia and/or chance of opportunistic infections. Especially the G-CSF (Granulocyte Colony Stimulating Factor, Filgrastim, "Neupogen") seems to be used routinely only after the fact, when neutropenia is demonstrated. Does cost have something to do with it, I wonder? I seem to recall, several insurance companies will cover the cost of stuff like Filgrastim only when the patient has demonstrable neutropenia. This may fall into the category of "Penny wise, Pound foolish", since the cost of hospitalizations due to infections after chemotherapy can be so much more expensive than prophylactic administration of neutrophil supporting cytokines like Neupogen.
The response rate is quite good, especially when considering the Fludarabine refractory bunch. I wish the toxicity was lower, after all that was the major reason for looking at Pentostatin instead of Fludarabine. (Bear in mind, there are so many differences between the various phase-2 clinical trials, it is next to impossible to have a strict apples-to-apples comparison). These results, combined with the Rituxan plus Pentostatin results discussed in the prior article gives us a rough idea of what to expect with an "RPC" (Rituxan, Pentostatin and Cyclophosphamide) combination. Nice to know there is a possible option for those that are refractory to Fludarabine, but I would not call this protocol hugely less toxic than the better known "RFC" protocol.
Journal of Clinical Oncology, Vol 21, Issue 7 (April), 2026: 1278-1284 © 2026 American Society for Clinical Oncology
Pentostatin and Cyclophosphamide: An Effective New Regimen in Previously Treated Patients With Chronic Lymphocytic Leukemia
Mark A. Weiss, Peter G. Maslak, Joseph G. Jurcic, David A. Scheinberg, Timothy B. Aliff, Nicole Lamanna, Stanley R. Frankel, Steven E. Kossman, Denise Horgan
From the Leukemia Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, and Cornell University Medical College, New York, NY; and the Greenebaum Cancer Center, University of Maryland, Baltimore, MD.
Purpose: Purine analogs and alkylators are important agents in the treatment of chronic lymphocytic leukemia (CLL). Previously, combinations of fludarabine and Chlorambucil were abandoned because of increased toxicity from overlapping myelosuppression and immunosuppression. Of the purine analogs active in CLL, pentostatin may be least myelosuppressive. We hypothesized that combining pentostatin with cyclophosphamide would have less myelotoxicity than combinations using other purine analogs.
Patients and Methods: We studied 23 patients with previously treated CLL. All patients received pentostatin 4 mg/m2. Seventeen patients received cyclophosphamide 600 mg/m2, and six patients received cyclophosphamide 900 mg/m2. Both drugs were administered on day 1 of each cycle, and cycles were repeated every 3 weeks for six treatments. Filgrastim, sulfamethoxazole/trimethoprim, and acyclovir were administered prophylactically. The median number of prior treatment regimens was three (range, one to five) with 13 patients (57%) refractory to prior fludarabine therapy.
Results: The cyclophosphamide 900 mg/m2 dose level was associated with moderate to severe nausea, and we chose cyclophosphamide 600 mg/m2 as the dose for further study. There were 17 responses (74%; 95% confidence interval, 63% to 85%), including four complete responses. The response rate was 77% in fludarabine-refractory patients. Myelosuppression was acceptable with grade 3/4 neutropenia and thrombocytopenia, seen in 35% and 30% of patients, respectively. The relative sparing of thrombopoiesis can be seen in that only one patient (5%) with an initial platelet count of more than 20,000 required platelet transfusions while receiving therapy.
Conclusion: Pentostatin 4 mg/m2 with cyclophosphamide 600 mg/m2 is safe and effective in previously treated patients with CLL. On the basis of these results, we are currently studying pentostatin, cyclophosphamide, and rituximab (PCR) therapy in patients with CLL. Supported in part by grants from Supergen, The Beatrice Renfield Foundation, The Michael Sweig Foundation, and the Archie W. and Grace S. Berry Charitable Foundation.

What Does It Mean to Be PCR Negative?
Date: 3/14/03
by Chaya Venkat
One of our more perceptive members raised a question about results from an FCR study reported by MD Anderson. She noticed that 57% of CR patients were PCR negative, but also that 36% of the NPR and 33% of the PR patients were also negative. The question she raised was - Can one achieve less than a complete remission and still be PCR negative?
A very good question indeed.
I looked up the ASH2002 abstract she queried. (It is attached below, for easy reference of our members. I present only the first four columns of the table in the abstract: those of you who want to see the whole enchilada will have to get it from the ASH abstracts site.
The member was absolutely right, the total of CR, NPR and NR percentages (67%, 19% and 18% respectively) add up to more than 100%. May be that is a simple typo, but what is even more puzzling is how it is possible to be PCR negative, with less than CR response. By definition, a "PR" means "Partial Response", with less than perfect cleaning out of the peripheral blood, bone marrow and lymph nodes. How can such a response qualify for PCR negative? I have struggled with this one all afternoon, trying to come up with more and more bizarre explanations that would fit the facts, and I have finally given up. May be I am just missing something important here, some pivotal concept I got garbled up, I don't know.
Any one going to Anderson in the next little while? How about doing us a favor and checking this out with any one of the many authors on this paper?
A High Proportion of Molecular Remission Can Be Obtained with a Fludarabine, Cyclophosphamide, Rituximab Combination (FCR) in Chronic Lymphocytic Leukemia (CLL).
Michael Keating, Taghi Manshouri, Susan O'Brien, William Wierda, Hagop Kantarjian, LaShonda Washington, Susan Lerner, Maher Albitar.
Leukemia, UT M. D. Anderson Cancer Center, Houston, TX, USA; Hematopathology, UT M. D. Anderson Cancer Center, Houston, TX
Definitions of complete and partial remissions (CR+PR) in CLL have become more stringent as treatments have become more effective. The NCI Working Group (NCIWG) categorizes patients (pts) as having CR, nodular (N) PR (CR except for residual lymphoid aggregates on bone marrow (BM) biopsy) and other PRs. Many CR pts have clonal disease detectable on flow cytometry and molecular remissions (PCR negative for IgVh gene) have been infrequently reported. A combination chemoimmunotherapy protocol has been developed (FCR) which combines fludarabine 25 mg/m2 per day for three days, cyclophosphamide 250 mg/m2 per day for three days, and Rituximab 375 mg - 500 mg/m2 on day 1 (Proc ASH 98:771a, #3210, 2026). The use of FCR as initial therapy for 135 CLL pts has resulted in 67% CR, 19% NPR, and 18% PR. Nine responders have relapsed clinically and 12/135 pts have died: 2/19 (2%) CR, 1/19 (5%) NPR, 4/18 (22%) PR and 5/7 (71%) Fail. Seventy- seven pts had PCR performed on BM at the end of therapy (usually 6 cycles) and 125 pts had flow cytometry performed. The relationship between NCIWG response, PCR and CD5 + 19 co-expression on flow, and likelihood of relapse is shown below.
|
NCIWG |
Total |
PCR- |
PCR Relapse |
|
CR |
91 |
35/61 (57%) |
11/35 (31%) |
|
NPR |
19 |
4/11 (36%) |
1/4 (25%) |
|
PR |
18 |
2/6 (33%) |
0/2 (--) |
CD5+19% co-expression < 1% occurred more frequently in pts < 70 years of age, spleen size < 5 cm, below the L costal margin, beta-2- microglobulin < 3 mg/L and lower marrow cellularity. No characteristics predicted for PCR negativity. The degree of PCR positivity is semi-quantitated by comparing the level of PCR amplification of IgVh to the ras gene and developing an IgVh/ras ratio. 13/41 PCR negative pts have become low level PCR positive usually within six months of follow-up. Nine of the 36 PCR positive pts have increased the ratio by more than 100% and 13 have had a > 50% decrease. None of the PCR negative pts have had a clinical or flow relapse while three of 36 pts have had a flow relapse and two a clinical relapse. Two of 85 pts (2%) with CD5+19 co-expression < 1% have had a flow relapse vs. 15/34 (44%) with 1% (P<.001). With longer follow-up, the better quality remissions obtained with FCR will allow exploration of the characteristics of response, which are the best predictors of prolonged survival.
Keywords: Chronic lymphocytic leukemia\ PCR negative\ Flow remissions
______________
The member who raised the question posted her solution of the mystery the next day. I would like to thank her for her detective work.
"I believe that I have figured out the actual RFC response percentages from 2026 ASH abstract by Dr. Keating et al. Looking at the breakout of the 12 people who died during this protocol and transposing the '2/19 2%CR' pts who died to 2/91, the results seems to be as follows:
Total Patients 135
Total Remissions 128 (94.8%)
Complete Remissions 91 (67.4%)
Nodal Partial Remissions 19 (14.1%)
Other Partial Remissions 18 (13.3%)
No Response 7 ( 5.2%)
This does now add up to 100%. There is a series of incorrect numbers and one transposed number on this abstract. I don't know who prepared it, but someone needs either a course in keying/proofreading or a basic course in Math.
Regardless, the remission numbers are certainly impressive and better than anything else I've seen. It will be interesting to see longer term results of this therapy."
The preceding raises an interesting question about PCR negativity and what it means. How can one have only a partial response, a lowly "PR", and yet be PCR negative, as in the above ASH abstract?
Here is what I came up with. I will discuss the science behind PCR (polymerase chain reaction) in another article. It may come as a surprise to some of you, not all the research labs agree on the exact protocol for how PCR tests ought to be run, the specific probes used and the specific markers looked for in the test. You might get quite different results, depending on the protocol used. But for purposes of following the logic of this article, it is enough to understand it is a technique that allows much more sensitive detection of cancer cells, to the tune of 1 in 100,000.
With that out of the way, let us see how it is actually used in monitoring CLL. First step, the researchers obtain a sample from the patient. Some researchers swear by peripheral blood, some go for the bone marrow. There are arguments to be made for both cases. Peripheral blood is reasonably homogeneous, that is it is the composition through out the body as far as lymphocytes are concerned. You are not likely to get a different WBC number if the blood is drawn from your left hand as opposed to your right hand, for example. There is not much chance of what is called "sampling error" in this procedure.
Not so with bone marrow. Marrow is a non-homogeneous material, it can vary from part of the bone to the next. Let us consider a patient with very little minimal residual disease, the few CLL cells left may be clustered in little nodules in different parts of the bone marrow. If this is the case, unless the bone marrow sample is obtained exactly from the location of one of these nodules, by sheer happenstance, the marrow will look clean. This type of sampling error is unavoidable in all cases where the sample is obtained from a non-uniform semi-solid. So, a BMB could give what is called a false negative, i.e., the marrow happened to look clean at the particular point from where the sample was obtained, perhaps right next to a tight little nest of CLL cells. Obviously, a positive is still a positive, if the BMB shows CLL cells, there is no way of avoiding the bad news: yup, you still have CLL cells in your bone marrow.
Given the possibility of sampling error in bone marrow samples, and no such error likely in peripheral blood, why does any one do PCR testing with bone marrow? Well, because in diseases like CLL, the ultimate action is always in the bone marrow. You could have squeaky clean peripheral blood, but if your bone marrow is still infiltrated with cancer, which is the only place where new red blood cells etc can be made, you don't have much to write home about. The analogy is the bone marrow is the factory where stuff is made, the circulating blood system is just the highways and traffic leading to and from the factory. If the factory produces the wrong stuff, or not enough of the right mix of products, you are still out of luck, even if there are no traffic jams on the highways.
Now for some mind-bendingly large numbers. A normal human with no CLL has about 5 billion B-cells in his body, give or take a few.
Some one with CLL and a WBC count of about 100K has about a hundred times more, say 500 billion CLL B-cells. If you undergo therapy that kills 99.99% of all the CLL cells, which was about the limit of prior generation detection technology, you could still have 50 million CLL cells left over in your body!!
Let us say you are in deep, deep CR, you have the approximately 5 billion B-cells of a normal person. PCR negativity means that they would catch it if you have one bad CLL B-cell in 100,000 perfectly good ones. Since there are 5 billion cells to contend with, you could have as many as 50 thousand CLL cells and still be declared PCR negative.
Quite an improvement in our measurement capability, the first case of 99.99% cell kill meant there could still be as many as 50 million CLL cells left over. PCR negative means that measurement sensitivity has increased to the point where we can say there can be at the most about 50 thousand CLL cells. Would you prefer 50 million CLL cells left over, or would you instead opt for 50 thousand CLL cells left over? The answer is obvious, it is better to be PCR negative than PCR positive. Right?
All this assumes several things: first, we assume the sample used to do the PCR testing was a representative sample, without sample error. This is particularly so in the case of bone marrow samples, and explains why it is possible to get PCR negative result from people who are only in PR!! There is clearly CLL in the patient's blood and perhaps lymph nodes, (which is why he/she is classified as PR) therefore a good bet there is some left over in the bone marrow as well, just that the sample of bone marrow that was used to do the PCR test happened to come from a spot that did not have any CLL. I do not see how one can be 100% certain there is no sample error in BMBs, except by testing all of your bone marrow. Small problem, if all of your bone marrow is extracted for doing the PCR test, you will have no marrow left, and you will die pretty quickly. That is a rather drastic way of making sure all of the CLL cells are killed.
In addition to the sampling error issue, there is also the whole statistics thing. Yes, PCR testing takes the sensitivity to new heights, but it does not give you a perfectly clean bill of health. Remember those pesky 50 thousand or so CLL cells that could still be there, and yet not trigger the PCR test? Well, the real question then is what happens to these final dregs of CLL cells that may still be there. Will they grow back to full fledged clonal population, enough to kick you out of remission, or with such low numbers of CLL cells will your body's defense systems be able to keep them in check indefinitely? That depends on the particular type of CLL clone you have got, its propensity to multiply and not die on command, and yes, the state of your immune system.
An interesting question: do people with PCR negative status obtained after deeply immune suppressive therapy relapse more often than those who got the PCR negative status after a relatively easy therapy that left their immune systems relatively intact, and able to take care of the remaining few cancer cells? Another aspect of the same question, are the few thousand CLL cells perhaps left over after heavy duty therapy more resistant to getting killed by the immune system, and therefore more likely to grow back?
One of the approaches discussed on this site several times is to go after minimal residual disease left over after RFC therapy, with low dose, subcutaneous Campath. Several studies have suggested that patients who were short of PCR negative after RFC could be kicked into PCR negative status after an additional treatment of Campath. Now Campath has a reputation for being quite immunosuppressive, killing many cell lines other than B-cells, for example T-cells and NK cells. If the PCR negative patient has just a few, 50 thousand or so, CLL cells left over after the RFC plus Campath treatment, does this also mean there is a chance the last few stragglers of CLL cells now have a carte-blanche to grow unchecked for a while, till the body's immune system has a chance to grow back and try to control their proliferation?
I will be the first to admit, there is very little information to answer many of these questions. It is only recently that CLL therapies have gotten to the point that PCR negative status is even a possibility after therapy. It has not been long enough time to get good statistics on survival rates. Will people with PCR negative status have significantly longer survival, statistically speaking, than those who are PCR positive? I think the answer to that will be "yes". But does that mean a specific individual patient who is PCR negative have a cast iron guarantee he will not relapse? Unfortunately, no such guarantee. In fact, we have already begun to see patients who were initially PCR negative right after RFC therapy who later became PCR positive, and eventually relapsed. The reverse is possible too: patients who did not make the PCR negative status right after therapy, i.e., had more than the expected bare minimum number of CLL cells left over, nevertheless continued to improve (maybe the residual effects of the therapy working slowly, may be the immune system doing its bit) and finally made the grade of PCR negative.
If you are confused by all this, join the crowd. For me, putting my money where my mouth is, I recommended the least toxic therapy option I knew, namely Rituxan as frontline and monotherapy to my husband, and initiated it soon enough to have a good chance of getting a good remission. For majority of patients who are good candidates for Rituxan therapy (see my previous articles on why not every one may not be a good candidate for this monoclonal therapy), I no longer feel watch and wait is the best option. W&W was a good choice in the past, when there were NO non-toxic therapy choices, none that did any good without costing you an arm and a leg, so to speak, in damaged immune system. Monoclonals like Rituxan have changed that scenario, at least for some of us. Earlier treatment means less chance for other complications to set in, such as autoimmune diseases, and earlier stage patients seem to respond better to Rituxan monotherapy, perhaps because the immune system is still pretty intact and able to work with the monoclonal. The downside is that I doubt people are likely to get PCR negative status with just Rituxan as frontline monotherapy, not unless we learn ways of enhancing its effects.
Oh yes, on the other subject: we are not making any efforts to find out if my husband's potential "CR" is also a "PCR negative". Most likely, since Rituxan was the only drug used, he is still PCR positive, but also hopefully, his immune system is sufficiently undamaged after therapy that the remaining CLL cells can be kept under control for a nice long time. That adds up to our hoping for a nice long remission, but I do have not much hope of a "cure". Only time will tell if today's PCR negative patients who have undergone more intense therapies are "cured". But the word "cure" begins to lose its meaning, in the context of prolonged and sustained remissions with good quality of life. I wish all our PCR negative friends long, uninterrupted remissions that last as long as their natural life spans. This article was not meant to devalue your hard won PCR negative status, in fact as I re-read it, it has more questions than answers.

Allopurinol
Date: 2/17/03
by Chaya Venkat
 Allopurinol is prescribed just prior to therapy in CLL patients. This is particularly the case if the patient has high tumor load. The logic is that if the tumor cells gets killed in large numbers and in a short period of time, the body has trouble getting rid of the debris. Especially the proteins in the dead cells have to be metabolized, leading to formation of uric acid. Uric acid has a limited solubility in urine, and if it builds up to high levels in the blood because too much of it is being produced for the kidneys to handle, the situation can be potentially very serious. Kidney failure is the worst case scenario, kidney stones down the road is a more long term worry.
Allopurinol is prescribed just prior to therapy in CLL patients. This is particularly the case if the patient has high tumor load. The logic is that if the tumor cells gets killed in large numbers and in a short period of time, the body has trouble getting rid of the debris. Especially the proteins in the dead cells have to be metabolized, leading to formation of uric acid. Uric acid has a limited solubility in urine, and if it builds up to high levels in the blood because too much of it is being produced for the kidneys to handle, the situation can be potentially very serious. Kidney failure is the worst case scenario, kidney stones down the road is a more long term worry.
In fact, we wanted to follow Dr. Hainsworth's advice, Allopurinol three days ahead of first infusion, and end it after that. Our local oncologist felt otherwise, that my husband should continue taking it for the duration of the four week infusion period. We decided to go along with her wishes. However, we did not reckon on the side effects: rash and itch.
By the time the second infusion rolled around, my husband had substantially reduced peripheral blood WBC, around 28K, and his uric acid level was at the low end of normal. So, when the itching started, it was a no-brainer to stop taking the Allopurinol. The rash (mild to start with) cleared within 24 hours, the itching was better, too, and was gone within 48 hours. No other medication was needed.
Some things you can do: the usual warning to stay well hydrated is really important, the greater the urine output, the greater the amount of uric acid that can be eliminated safely and naturally. But it is important to take the Allopurinol 3 days ahead of time for the first infusion, especially if the counts are high. Keep an eye out for rash/itching, discontinue Allopurinol if either becomes a problem.

Date: 12/2/02
by Chaya Venkat
Very often, in medical literature and on patients charts, the dosage of drugs to be administered is given in terms of so many units per meter square. For example, the now standard dosage for Rituxan is 375 milligrams per meter square at one infusion. I thought I would take some of the mystery out of this, so that in future you can calculate for yourself how much of the drug you should be getting. Or your significant other can do it for you, while you are dealing with infusion related side effects!
Chemotherapy drugs (and others too, I suppose) are given on the basis of a persons "BSA" or "Body Surface Area". The units are meter square. Sort of makes sense, if you are a large person, with a large BSA, you should be getting more of the drug than a tiny little old lady half your size. So, how do you calculate your BSA? The URL below gives you the detailed scoop. You can visit that, or you can use this simple 3-step method:
1. Multiply your weight in pounds by your height in inches,
2. Next, divide by 3131,
3. Then take the square root of the answer.
The result is your BSA. Average BSA's for men are around 2.0, women have lower BSA's, around 1.6. A 9 year old child may have a BSA around 1.0
Here is an example: let us consider an average man of 5' 10", who weighs 180 pounds.
So, the calculation goes like this: 70inchesX180 pounds /3131=4.02 and the square root of that is 2.0.
So, using our Rituxan dosage above, this guy should get 375 X 2 = 750 milligrams .
Link: Calculation of BSA - Formulas from the BC Cancer Agency
Editor's Note: If computing square roots on a calculator is not your bag, you may find it more convenient to use the BSA calculator in our Reference section.

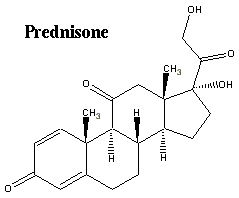
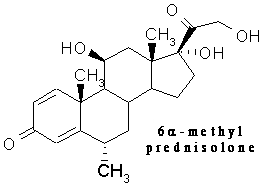
Prednisone vs. Methyl Prednisolone
 |
 |
Date: 12/2/02
by Chaya Venkat
This clarification from a friend was most useful to me in understanding better the dosages of methylprednisolone mentioned in the Kipps HDMP + Rituxan trial:
"Methyl Prednisolone has a different make up than Prednisone. 40 mg to 2 grams is the range of dosage. 1 gram is a high dosage. Right off the cuff a common dosage for the Methyl Prednisolone was 500 mg. Prednisone is a mineral corticoid. Methyl Prednisolone is a gluco-corticoid. Gluco-corticoid doesn't have as dramatic side effects as mineral corticoid."
Bottom line, if 1 gram per day of the methylprednisolone is a high dosage, the 2 grams per day that we calculated for our average male patient in my articles yesterday is a mega dose indeed. I am also a little taken aback that they did not taper off the administration of the corticoid more gradually. But perhaps they did, and that detail did not make it into this presentation.
Anyone out there with more experience with this specific gluco-corticoid, help us out here, share your knowledge and experience with us.
The following is an link to a web page discussing the structural and functional differences between gluco- and mineralocorticoids. It also identifies why the systemic use of either variety is approached with caution.
http://www.people.vcu.edu/~urdesai/adrn.htm

Date: 11/22/02
by Chaya Venkat
 In view of the seriousness of this subject, and to sincerely discourage anyone thinking of self-medicating themselves with corticosteroids without medical supervision, I have cobbled together the following on prednisone.
In view of the seriousness of this subject, and to sincerely discourage anyone thinking of self-medicating themselves with corticosteroids without medical supervision, I have cobbled together the following on prednisone.
Prednisone is a synthetic hormone similar to hydrocortisone, a natural hormone produced by the adrenal glands. It belongs to a large group of similar drugs known as corticosteroids, which are prescribed for literally hundreds of conditions. It is usually given in tablet form but can be given intravenously when necessary. The drug usually is used in combination with other agents to suppress the immune response. It acts by decreasing swelling and inflammation of tissues.
Because rheumatoid arthritis (RA) is a disease which involves the immune system attacking and destroying joints, drugs that prevent immune responses in the body and decrease inflammation have been utilized in the treatment of this disease. The class of medications capable of these actions is called corticosteroids or "steroids". These drugs are different from the male hormone-related compounds (anabolic steroids) that athletes sometimes abuse.
The downside with prednisone is its many side effects. The higher the dose or the more times you have been treated for rejection, the more side effects. As the dose is gradually reduced, side effects diminish. In most cases, prednisone can be reduced safely. Also, use of combinations of drugs has allowed physicians to prescribe lower doses of prednisone than in the past. Regardless of complications, never stop taking prednisone unless you are instructed to do so by your doctor. Many problems can occur if prednisone is stopped suddenly.
Prednisone, a corticosteroid, is similar to a natural hormone produced by your adrenal glands. It often is used to replace this chemical when your body does not make enough of it. Prednisone decreases inflammation or swelling by preventing certain inflammation- promoting chemicals in the body from moving into the affected area (e.g., airway with asthma or skin with poison ivy).
Prednisone also suppresses your immune system so that your entire body is affected. In fact that is the basis of its use in CLL, since it suppresses the proliferation of B-cells. It is also used in controlling ITP (idiopathic thrombocytopenia), where the platelets are attacked and destroyed by T-cells that are out of control and acting inappropriately.
Prednisone is a synthetic steroid hormone. It cannot be stopped abruptly because the adrenal gland, which makes natural steroid hormones for the body, is suppressed by long term prednisone administration. Since some steroids are necessary for life, abruptly stopping prednisone leaves one without any steroids at all, a condition known as Addisonian crisis.
Given time, if the adrenal is stimulated to produce steroids by gradual reduction in the dosage of prednisone, it will eventually begin to wake up and produce natural steroids in most cases. It is thought that if one becomes ill, more steroids are needed since the natural response to stress (like trauma, an operation, an infection, etc.) is for the adrenal gland to pour out steroids. Until one's adrenal glands are up to par though, this is not possible. So anyone on steroids or recently weaned off steroids needs to be aware of this.
To repeat myself: do not suddenly stop systemic steroids; your doctor will explain how to gradually come off them (particularly important if you have been on them for more than six weeks). Discuss any side effects you may experience with your doctor.

Bryostatin plus Fludarabine
Date: 11/22/02
by Chaya Venkatt
 Here is one more of these chemo combinations: the results are less than impressive but the investigators seem to conclude otherwise in their wrap-up. Given the results from the reported clinical trial, here is another combination whose value I just do not understand. Just plain don't get it.
Here is one more of these chemo combinations: the results are less than impressive but the investigators seem to conclude otherwise in their wrap-up. Given the results from the reported clinical trial, here is another combination whose value I just do not understand. Just plain don't get it.
Here is the skinny: "progressive CLL patients" (does not necessarily mean refractory or relapsed cases, just those with increasing WBC, which means just about all of us!) were treated with Fludarabine (maximal dose) plus escalating doses of Bryostatin till toxicity limits were reached. Arms 1 and 2 of the study differed by just which of the drugs was given first, which was given second.
Side effects included neutropenia, which represented the primary dose limiting toxicity. Cumulative myelosuppression, particularly lymphopenia was also frequently observed. This was not a "free lunch" protocol by any stretch of the imagination. One wonders exactly how much cumulative bone marrow damage the patients absorbed, after going through the full protocol, and how long it took them to recover from it.
OK. Now for the reward you get if you went through this process. If you were a CLL patient, as seen in the table below, out of 19 patients who were enrolled in this study, there were zero complete remissions, 5 partial remissions, for a grand total of 26% total responders of any kind. To my uneducated mind, plain Fludarabine would do better than this, without the added toxicity of Bryostatin!
Guess what, I must be missing something here, I expect I am just not smart enough to spot the point of it all, because the researchers feel that based on these results, "the Phase II evaluation of this drug combination in CLL and indolent NHL appears justified". That is a direct quote from the ASH abstract below. Any volunteers for the Phase-2 trials?
Every once in a while, one of the major consortium docs gets up on the soap box, bemoaning the low participation rates in clinical trials. Patients should be braver, they say, pave the way for future generations. I am all for it. But, guys, how about a bit more transparency, and critical evaluation of the prior phase results, before you ask us to put our bodies on the line? I fully endorse and support participation in clinical trials, if it gets us closer to better therapies down the road. But I most emphatically would not want my guy to participate in a boondoggle trial, ginned up for getting more grant money or research publications.
We are missing a crucial piece of the puzzle here, in how clinical trials are defined and conducted, especially when they deal with real live patients. And that crucial piece of the puzzle, in my opinion, is patient participation on clinical trial advisory boards, internal review committees, and ethics boards. Patient advocacy and awareness forces a healthy reality check on these studies. How informed, and I mean truly informed about the risk and rewards, are the average participants in clinical trials? That is what CLL Topics is all about. Information, empowerment and participation. Together, we can make it better for all of us. But we do have to get heard.
Phase I Trial of Bryostatin 1 (NSC 339555) and Fludarabine in Patients with Chronic Lymphocytic Leukemia and Indolent Non-Hodgkin's Lymphoma.
John D. Roberts, Eric J. Feldman, Mitchell R. Smith, Steven Grant.
Massey Cancer Center and the Department of Internal Medicine, Virginia Commonwealth University, Richmond, VA, USA; Department of Medicine, Weill Medical College of Cornell University, New York, NY, USA; Lymphoma Service, Fox Chase Cancer Center, Philadelphia, PA, USA
Previously we demonstrated that treatment of leukemic cells with the macrocyclic lactone protein kinase C activator/down-regulator bryostatin 1 following exposure to the purine analog fludarabine synergistically enhances apoptosis and antiproliferative effects (Leukemia 13:1046,1999). However, other studies have shown that the sequence bryostatin followed by fludarabine leads to optimal therapeutic effects in a murine CLL xenograft model (Int J Oncol 14:945,1999). To assess the dose limiting toxicities and to identify the MTD for each sequence, two multi-institutional phase 1 trials have been initiated in patients with progressive CLL and relapsed/refractory indolent NHL. Patients have received escalating doses of bryostatin 1 via 24h continuous infusion followed by fludarabine (maximal dose 25 mg/m2) daily for 5 days (Arm 1), or fludarabine daily for 5 days followed by bryostatin via 24h continuous infusion (Arm 2). Both regimens have been well tolerated with neutropenia representing the primary dose limiting toxicity. Cumulative myelosuppression, particularly lymphopenia, also has been observed. A total of 53 patients have been entered to date. Arm 2 has been completed with recommended Phase II doses of 25 mg/m2 for fludarabine and 50 mcg/m2 (bryostatin 1). Arm 1 is nearing completion, with patients now entered at the 50 mcg/m2 bryostatin 1 dose level. Of the evaluable patients to date, 17 responses (32%) have been observed. These have occurred in CLL and NHL patients entered on both arms of the study (Table 1), including responses in some heavily pre-treated patients or those who have received prior fludarabine therapy (6 patients). There has been no clear therapeutic superiority for one sequence, although there appears to be a slight trend in favor of Arm 2. Arm 1 will continue to accrue until the MTD is identified. Correlative laboratory studies revealed variable in vivo effects of bryostatin 1 on the ex vivo response of CLL cells to fludarabine, although bryostatin 1 itself appeared to induce apoptosis in cells obtained from a subset of patients. These findings demonstrate that a regimen combining bryostatin 1 and fludarabine is well tolerated and has activity in CLL and indolent NHL. Based upon initial results, Phase II evaluation of this drug combination in CLL and indolent NHL appears justified.
| Arm 1 Patient Responses | Arm 2 Patient Responses | ||||||
| Patients | CR | PR | Patients | CR | PR | ||
| CLL | 14 | 0 | 5 | CLL | 5 | 0 | 0 |
| NHL | 11 | 0 | 5 | NHL | 23 | 3 | 4 |
I guess my beef with this particular study, and that is too strong a word for my concern, is that even with these less than exciting results, the "spin" at the end of the abstract is that more trials are justified along these lines. How about calling a spade a spade, these are researchers and doctors, not politicians and lawyers that have to declare every new venture a thundering success. Clearly, not all clinical trials and reports are guilty of this, my comments are about this particular abstract. Also, it was not clear from the description of the patient group that the CLL patients were indeed a far gone bunch with no other options. They were described as having "progressive disease", which is every patient with increasing WBC. The NHL patients, on the other hand, were clearly spelled out as refractory and resistant to prior chemotherapy. If indeed the CLL patients selected for the study were also similarly handicapped, I would expect the researchers would have made that clear. If patients had other options, then the results of this trial were truly unfortunate.
I am aware of the hope of transforming CLL to Hairy cell leukemia by use of Bryostatin, and that in fact the transformation did not happen along those lines. Would there be a follow-up of these patients who participated, to see if their un-anticipated transformation was not into something more aggressive and resistant than CLL? Anyone willing to take a bet with me there will be no long term study and monitoring results reported on how these patients fared down the road?
My main point is this: medical jargon is hard enough to understand, but we accept it is a language that is necessary to communicate effectively between researchers, so we lay people will do our best to learn it, live with it. We do our bit to explain the jargon. But there is no excuse for obfuscating statistics or "spin" on the results.
I think every ethics committee out there will agree with me, transparency in reporting the results is a primary responsibility of the researchers, and patients who are volunteering for these trials have an absolute right to expect it. It goes to the heart of the whole concept of informed consent, consent that is truly informed. I see a lot of Phase II & III trials listed on the official sites, that are looking to recruit participants. Very few of them clearly discuss the results of the prior trials conducted using the same approach. A few give references to scholarly articles, that are written to communicate with other researchers, not patients. There seems to be a pervasive assumption that participation in clinical trials is a matter of trust and faith, not understanding.
I recognize there are limitations on how much of the complex science we can follow, just because it is so complex. But the limitation should not be because of a lack of effort to communicate with patients. Trust and respect, these need to be two-way streets. Patients who participate in clinical trials are doing their community a huge service. They need every available resource in order to make informed decisions, and I for one believe strongly that they deserve the extra effort at straightforward communication.

Pentostatin Combination Therapy
Date: 9/23/02
by Chaya Venkat
 A member raised a question on indications in the literature that pentostatin/Nipent is less immunosuppressive than fludarabine.
A member raised a question on indications in the literature that pentostatin/Nipent is less immunosuppressive than fludarabine.
This is an excellent question, I wish I had an excellent answer to match. I have looked high and low for concrete information on clinical trial experience and results with this combination, because it would be nice to have this as an alternative to the "RFC" combo treatment.
However, It seems that the RFC has gotten a huge head start, and it is hard to fight the band wagon effect. There is now so much information on this combination, so many different trials at so many institutions, it is going to be hard to match that. Clinical trials cost a lot of money and it takes a lot of time and effort besides money to develop the kind of track record RFC has developed. So, unless the trial at Memorial Sloan Kettering comes up with significantly better results than those coming out of Anderson with RFC, I am afraid this train has left the station.
That is too bad for us patients, who would have benefited from having a choice. Pentostatin might indeed be better for some, compared to fludarabine. But even before the era of the combos, Fludarabine was far more often prescribed than Pentostatin, even though both are purine analogs, very similar in structure and discovered about the same time.

Dexamethasone and Other Drugs
Date: 10/28/02
by Chaya Venkat
 Here is a link that has a lot of information about a wide variety of drugs, their uses, contra-indications, dosages, side effects, toxicity etc. It is a commercial site, and you have to turn a blind eye to the many advertisements cluttering up the screen, but it is a quick way to get basic information on many of the drugs we come across. Suggest you file this URL someplace where you can find it again next time you need to look up something.
Here is a link that has a lot of information about a wide variety of drugs, their uses, contra-indications, dosages, side effects, toxicity etc. It is a commercial site, and you have to turn a blind eye to the many advertisements cluttering up the screen, but it is a quick way to get basic information on many of the drugs we come across. Suggest you file this URL someplace where you can find it again next time you need to look up something.
URL: http://www.rxlist.com/cgi/generic/dexameth_cp.htm

Flavopiridol in in Fludarabine Refractory Patients
Date: 9/22/02
by Chaya Venkat
 Recently, we have been discussing cutting edge immunotherapies that may some day become available. Meanwhile, the reality of the situation is that a very substantial percentage of CLL patients are not struggling with making first therapy decisions, but trying to come to terms with life after several rounds of chemotherapy.
Recently, we have been discussing cutting edge immunotherapies that may some day become available. Meanwhile, the reality of the situation is that a very substantial percentage of CLL patients are not struggling with making first therapy decisions, but trying to come to terms with life after several rounds of chemotherapy.
Fludarabine is still the gold standard drug for CLL. Unfortunately, the majority of patients either do not attain complete remissions, or worse, fail to respond to Fludarabine. Furthermore, all patients with CLL treated with Fludarabine eventually do relapse, and most eventually do develop resistance to this drug. Prospects are poor for a good response even with combo treatments like RFC if your disease is already refractory to Fludarabine.
So, this article is devoted to a new drug on the horizon, called Flavopiridol, that may give this population of patients another fighting chance. That's what this disease is all about, living to fight another day, living till the incremental breakthroughs add up enough to be sufficient to define a cure.
Several prognostic factors are associated with drug resistance, including abnormal p53 function, over-expression of bcl-2, and incubation of tumor cells with interleukin-4 (IL-4). You can read more about genetic aberrations and what they mean in our "Files" section.
Of these, the most extensively studied, uniformly predictive factor for poor response to therapy and inferior survival in CLL patients is aberrant p53 function, as characterized by point mutations or chromosome 17p13 deletions. Indeed, virtually no sustained responses to either Chlorambucil or Fludarabine based therapy have been documented in multiple single institution case series for those CLL patients with abnormal p53 function.
What if we can find a drug that overcomes the effect of the p53 mutation, and thereby overcomes the resistance that CLL cells have developed to available chemotherapy drugs? I have come across a truly excellent and detailed article in Blood magazine that describes the mode of functioning of flavopiridol, a novel synthetic flavone currently entering phase II trials. It demonstrates marked cytotoxicity toward human CLL cells and may allow us to get around either IL-4-induced resistance or that incurred by a p53 mutation.
If you are so inclined, do look through the full article. Unfortunately, it is written for an audience of experts, not patients.
Flavopiridol Induces Apoptosis in CLL (full article in Blood).
There is still a great deal of debate on exactly how flavopiridol works. Some feel that it works by down regulation of the onco-gene bcl-2, which makes it harder to kill CLL cells. Reducing the amount of this cancer protecting protein will obviously help the apoptosis process (kill rate of cancer cells). Others, including the authors of this paper, fell that flavopiridol has significant in vitro activity against human CLL cells through activation of caspase-3, which seems to have very little to do with down-regulation bcl-2 modulation, the presence of IL-4, or p53 status. For our present purposes, I am going to ignore the "how" and focus on "so what does it do for us" aspect of it. To make a long story short, I will just do bullet points for this article, perhaps expand later if anyone is interested.
Flavopiridol acts very quickly. In-vitro experiments show that it is cytotoxic toward CLL cells with an optimal exposure period of 24 hours
Flavopiridol Is equivalently cytotoxic to untreated and previously treated B-CLL cells. Sensitivity to flavopiridol is not altered by prior treatment and more advanced disease. It also does not seem to depend on whether or not the patient is already refractory to Fludarabine, the presence of a p53 aberration, or IL-4 incubation.
Flavopiridol is also cytotoxic toward normal mononuclear cells. In fact, comparison of the therapeutic index between B-CLL cells and normal mononuclear cells demonstrated no significant difference. What does this mean? This finding suggests that flavopiridol might produce adverse effects on normal lymphocytes and a heightened awareness for possible opportunistic infections should be present in these studies. This drug is certainly not for fun or recreational use!
Flavopiridol induces apoptosis in CLL cells very quickly. Unlike fludarabine, which induces apoptosis only after 1 day of exposure flavopiridol produced apoptosis as quickly as 4 hours .
Here is what the authors say at the end of it all:
"In summary, we conclude that flavopiridol has marked in vitro activity against human CLL cells at concentrations that are readily attainable in the clinic with the optimum time of drug exposure of approximately 1 day. Contrary to previous reports, flavopiridol exposure does not appear to modulate bcl-2 expression in human CLL cells but does result in activation of caspase-3. Unlike other effective agents currently used in the treatment of CLL, flavopiridol induces cytotoxicity independent of p53 status or IL-4 incubation, making it an ideal agent for targeted therapy toward high-risk subsets of patients with this disease. These findings justify early introduction of previously treated patients with CLL into phase I studies of flavopiridol using a 24-hour infusion schedule"
No cake walk here folks, but an option is always better than a kick in the head, says my commonsense.
To answer a question that was raised on the above article on Flavopiridol, once again, the devil is in the details. Flavopiridol has significant toxicity profile, for example it kills all lymphocytes, good bad and indifferent, with no selectivity at all for just the cancer cells. It is something to think about and discuss with one's doctor after all other chemotherapy avenues have been exhausted, or if for some reason there is good reason to expect that the patient is fludarabine refractory. It is a long way from being added to combo therapies such as RFC in clinical trials.
I guess if I had a DISC Assay test done, and it showed unequivocally that I would not respond to Fludarabine, and I had an aggressive form of CLL that mandated treatment of some sort right away, I would start thinking about including Flavopiridol, but not until then.

Novel Drug Delivery System
Date: 7/27/02
by Chaya Venkat
INEX is a Canadian company that may have an interesting way of delivering drugs right to the tumor. INEX's proprietary drug delivery system is called Transmembrane Carrier System (TCS).
To put it into very simple terms, the drug of choice, typically a drug that has already been approved and proven effective against the cancer in question, is encapsulated inside a lipid membrane (think of it as a fat globule, with the drug inside). Once injected into the blood stream, this globule is too large to pass through the walls of normal blood vessels. However, we all know by now that tumors grow by creating their own blood vessels (angiogenesis) to fuel their growth. But since tumors are usually in a hurry to get this done, and don't particularly care whether the job is done right, tumor vasculature is pretty shoddy workmanship, and there are all sorts of leaks and holes in these jury rigged tumor blood vessels. The fat globules containing the drugs can pass through these "leaky" walls of tumor blood vessels, right into the tumor tissue. The quotes below are from the web site of the company. The press release below reports some encouraging results of phase-2 clinical trials for aggressive NHL, conducted at M. D. Anderson.
Here is a concept that may well increase efficiency of drugs such as Rituxan. If you remember, Rituxan does a super job of clearing out the CLL cells in peripheral blood, but has a harder time clearing out the lymph nodes and bone marrow. Part of the problem is figuring out how to get sufficiently large dose of the drug into the lymph nodes and bone marrow, without escalating the dose to toxic levels for the rest of the body. Inex's approach may offer a solution.
"In developing targeted chemotherapy products, INEX combines already approved anticancer drugs with its proprietary TCS technology to make new drugs that are more effective with fewer side effects."
"The TCS technology permits the loading of a high concentration of an anticancer drug in the lipid envelope, provides extended circulation in the bloodstream, promotes accumulation of the drug in tumors and prolongs the release of the drug at tumor sites."
"Compared with free-form drugs, TCS has been shown in preclinical studies to deliver more of the cancer drug to a tumor site over a longer period of time, thus increasing the effectiveness of the drug, while also reducing side effects in healthy, non-targeted tissues." "This targeted approach is in contrast to how free-form (unencapsulated) drugs work. Free-form drugs circulate indiscriminately throughout the body, diluting the drug's effectiveness and causing toxic side effects to the patient's healthy tissues."
For immediate release: May 21, 2026
VANCOUVER - Inex Pharmaceuticals Corporation ("INEX"; TSX: IEX) presented interim results of an ongoing clinical trial today at the annual meeting of the American Society of Clinical Oncology in Orlando, Florida that demonstrate the potential of the company's lead product Onco TCS in combination therapy for the first-line treatment of aggressive non-Hodgkin's lymphoma (NHL).
"Interim results from 26 patients treated in the Phase II trial being conducted at The University of Texas M. D. Anderson Cancer Center in Houston, Texas showed that all patients responded to the treatment. Of the 26 patients, 25 (96%) completely responded to therapy and had all of their tumors eliminated and one patient (4%) partially responded to therapy and had their tumor volume decreased by more than 50% for an overall response rate of 100%."
"The current standard first-line treatment for the aggressive form of NHL is the CHOP chemotherapy combination, comprising the drugs cyclophosphamide, doxorubicin hydrochloride, Oncovin® (vincristine) and prednisone. The INEX trial is treating NHL patients with CHOP in which the Oncovin® (vincristine) component is substituted with Onco TCS, which is vincristine encapsulated inside INEX's proprietary liposomal drug delivery technology, Transmembrane Carrier System (TCS). Patients diagnosed with B-cell lymphoma also receive Rituxan® (rituximab), a monoclonal antibody, as recently published results support its investigational use with CHOP."

Does Chemotherapy Cause Transformation?
Date: 7/21/02
by Chaya Venkat
The abstract quoted below addresses one aspect of transformation in histopathology in lymphoproliferative diseases in general.
Basically, the authors looked for patients with "LPD" (i.e., lymphoproliferative disease in general, not necessarily CLL) who have had transformation to large cell form. Of the nine such patients they found, looking back at their therapeutic history, eight out of the nine had had fludarabine (some of them also had Rituxan), and only one had had only Rituxan, no fludarabine. Even if one accepts the argument that the transformations were due to the therapy, you may logically draw the conclusion that fludarabine is eight times more likely to cause transformation to more aggressive disease than Rituxan!! However, many more patients have undergone Fludarabine treatment than Rituxan treatment, making any such conclusion questionable.
There is some tentative evidence that during the period immediately after therapy, when the body's own immune system is not working well, it may be possible for secondary cancers to take root, or the original malignancy may transform into a more aggressive form. But the cause and effect of this hypothesis is hardly proven, and it rates more as a possible explanation. Actually, the authors themselves state: "This switch in the histopathology could merely reflect the natural course of progressive lymphoma and be unrelated to the therapy given." See what I mean, "lies, damn lies and then there is statistics?" The devil is in the details, all too often.
European Journal Of Haematology. Volume 68 Issue 2 Page 80 - February 2026.
Large-cell transformation of chronic lymphocytic leukemia and follicular lymphoma during or soon after treatment with fludarabine-rituximab-containing regimens: natural history- or therapy-related complication?
Yossi Cohen1, Nael Da'as1, Diana Libster1, Gail Amir2, Alan Berrebi3,Aaron Polliack1.
Novel therapeutic regimens containing purine analogs and monoclonal antibodies have led to significant improvement in the course of indolent lymphoproliferative diseases (LPD). Complete clinical and even molecular remissions have been achieved in an increasing proportion of patients. In parallel to their tumor cytotoxic effect, these agents are inevitably associated with prolonged immunosuppression inherent to their mechanism of antilymphocytic activity. Until now, attention has been paid mainly to opportunistic infection occurring as a result of the above drug-induced immunosuppression and less to other possible complications, such as malignancy or tumor progression in the immunocompromised host. Here we briefly report nine patients with previously treated indolent LPD in whom the onset of large-cell transformation occurred during or shortly after the initiation of regimens containing these agents before transformation occurred. One patient had received rituximab alone, three fludarabine-containing regimens and five received sequential regimens containing both agents. This switch in the histopathology could merely reflect the natural course of progressive lymphoma and be unrelated to the therapy given. The onset of this complication during, or so soon after, administration of these regimens seems to us more than just a chance relationship in these cases; however, we have no conclusive evidence to prove this hypothesis definitively. We draw attention to this phenomenon.

Thalidomide and Its Side Effects
Anti-angiogenic Agent
Date: 7/18/02
by Chaya Venkat
 As for thalidomide, I think the jury is still out, but there is a growing body of evidence that it may play an important role in angiogenesis prevention. Some of the more elegant anti-angiogenetic drugs such as Endostatin and angiostatin etc are not available on the open market, they are also very expensive. Thalidomide may prove to be the poor man's Endostatin. Part of the problem is that it is a cheap, off patent drug, and no one stands to make a lot of money pushing its therapeutic use in cancer treatment.
As for thalidomide, I think the jury is still out, but there is a growing body of evidence that it may play an important role in angiogenesis prevention. Some of the more elegant anti-angiogenetic drugs such as Endostatin and angiostatin etc are not available on the open market, they are also very expensive. Thalidomide may prove to be the poor man's Endostatin. Part of the problem is that it is a cheap, off patent drug, and no one stands to make a lot of money pushing its therapeutic use in cancer treatment.
Thalidomide was routinely prescribed as a "safe" sedative to pregnant women during the 50's and 60's. There were few side effects, except the now infamous "thalidomide babies" problem. Prevention of angiogenesis during pregnancy does horrid things to a developing fetus, and thalidomide is an effective angiogenesis inhibitor! Now researchers are learning how to turn this to our advantage, by inhibiting angiogenesis and thereby controlling progression of cancer. For the moment, the jury is out on the usefulness of this drug in CLL.
From member correspondence it is evident that at least one oncologist is concerned about the general side effects from 'closing off' the blood stream to the tissues in the rest of the body. He considered these side effects to be more dangerous (or may be just worse) than the disease.
From what I have read thus far, thalidomide is one of the most heavily researched drugs. Possibly because of the tragedy in the late 50's and 60's, causing the birth of the "thalidomide babies", this drug and its side effects has been gone over with a fine toothed comb. Besides its dangerous effects on pregnant women, it is considered to be a very safe and dependable drug, very well tolerated and with very little toxicity. Its major use (prior to its new debut as a cancer drug) was as an effective sedative and sleep inducer. I have not come across any other side effects (for non-pregnant and non- lactating women). You guys out there, this one has few worries for you. The major question seems to be one of level of effectiveness as an anti-angiogenesis agent, and only time will tell on that issue.
 Incidentally, anti-angiogenesis drugs such as thalidomide are not known to have any effect at all on existing, established vasculature, only on new and somewhat unstable blood vessels created by cancer. There may be some effect on wound healing, so it is not recommended that one be on anti-angiogenesis therapy just prior to major surgery, for example.
Incidentally, anti-angiogenesis drugs such as thalidomide are not known to have any effect at all on existing, established vasculature, only on new and somewhat unstable blood vessels created by cancer. There may be some effect on wound healing, so it is not recommended that one be on anti-angiogenesis therapy just prior to major surgery, for example.
I will look some more to see if there is additional information on side effects.
I am convinced that if you are not in the market for standard chemotherapy drugs, or the newer monoclonals like Rituxan and Campath, thalidomide therapy may be one option that is realistically open to you. There is also supposed to be a network of doctors that will work with their patients in designing individual cancer therapy protocols that use thalidomide, sometimes in combination with the so-called metronomic approach (extremely low dose chemotherapy, that is continuously administered as maintenance medication, usually orally, with much, much lower side effects). This is possible since thalidomide is a drug that is readily available, as opposed to other anti-angiogenesis drugs like Angiostatin and Endostatin that can only be obtained as part of a established research protocol. There are supposed to be many such "off-label" clinical trials underway at present, certainly for the better known solid tumors. I would love to get my hands on any and all information on this subject, as it pertains to CLL.

Conventional Chemotherapy vs. Metronomic Dosing
Date: 6/15/02
by Chaya Venkat
The link below provides some easy to understand details of the logic behind low dose chemotherapy treatments, also called metronomic therapy. I have excerpted some of the information at this site, for your convenience. The website, which is an individual's report of her fight with breast cancer, also lists some other technically more detailed references that you may wish to read. These are all complex issues, and it is difficult to "put them in a nutshell" so to speak.
http://www.geocities.com/lowdosechemo/
Conventional Chemotherapy tries to kill tumor cells. "Metronomic Dosing" kills the endothelial cells that feed the tumor cells.
Tumor cells are very adaptable. They change and mutate quickly, proliferate rapidly, and become drug resistant eventually. That is why some people are given one chemotherapeutic agent after another, with limited success, until they run out of options.
Endothelial cells proliferate much more slowly. They don't mutate like tumor cells and they die with much less chemotherapy than tumor cells. Endothelial cells are the life support for the tumor.
Conventional Chemotherapy is usually given on "day one" and then three weeks later on "day twenty-one." Your body is dosed with a "standard dose" on day one. This will kill some tumor cells, and kill a lot of the endothelial cells. But by day "twenty one" the tumor cells that didn't die will still be there mutating and becoming drug resistant and the endothelial cells will have grown back and be feeding the tumor again.
"Metronomic Dosing" therapy means giving ¼ of the Standard Dose of Chemotherapy (one fourth of what you would have received on day one) and dividing that dose over the twenty-one days. You get a little bit of chemotherapy everyday. This amount will not kill tumor cells, but it is enough to weaken and kill off their life support, their blood supply, which is made up of the endothelial cells.
Some low dose chemotherapeutic agents that have been used in the "metronomic dosing": Cyclophosphamide, Vinblastine, Gemcitibine, Taxol, Temedor.
Another approach to metronomic therapy depends on the fact that while conventional chemotherapy only uses a chemotherapeutic agent at conventional (high) dose, metronomic chemotherapy is often used in combination with other agents such as a COX-2 inhibitor or an anti-angiogenic agent.
COX-2 is short for the enzyme known as "cyclooxygenase-2". This enzyme promotes inflammation and blood flow. In recent studies, a number of groups agree that COX-2 is crucial for tumor angiogenesis.
Article from Journal of Clinical Investigation (Free full-text — J Clin Invest, June 2026, Volume 105, Number 11, 1511-1513)
A COX-2 inhibitor inhibits or prevents blood flow. Examples of Cox-2 inhibitors are Nonsteroidal anti-inflammatory drugs (NSAIDs) such as the following agents and their OTC brands: ibuprofen (Advil®), naproxen (Aleve®; or Naprosyn®), aspirin (Bayer®), acetaminophen (Tylenol®).
Prescription names include: celecoxib --Celebrex®, diclofenac -- Voltaren®, etodolac --Lodine®, fenoprofen -- Nalfon®, indomethacin -- Indocin®, ketoprofen - Orudis®, Oruvail®, ketoralac --Toradol®, oxaprozin -- Daypro®, nabumetone -- Relafen®, sulindac -- Clinoril®, tolmetin -- Tolectin®, rofecoxib -- Vioxx®.
Simply put, angiogenesis (angio'gen'esis) is the growth of new blood vessels - is an important natural process occurring in the body, both in health and in disease. An anti-angiogenic agent prevents the growth of new blood vessels or attacks the existing blood supply (in this case to tumors). Examples of Anti-angiogenic agents used so far in metronomic dosing are: Thalidomide, DC101, TNP470, Endostatin.

Chemotherapy and Cognitive Impairment
Date: 5/18/02
by Chaya Venkat
I know that for many patients an issue of concern is cognitive impairment arising from chemotherapy. Most doctors like to deal with the physical side effects, such as pain and nausea. Mental stuff is easy to sweep under the carpet, or dismiss as "it is all in your head, dear". Come to think of it, American hospitals have not been that good at dealing with pain either, but that is the subject for another day.
There have now been several small but quite rigorous studies done that seem to confirm what patients have known for a long time: there does seem to be some lingering effects of chemotherapy on how the brain functions. Not surprising, really, if you think about it. Not much is known on whether this is a temporary phenomenon or a lasting one. Also unknown is whether the newer therapies such as monoclonal antibodies have similar effects. At the bottom of this article is a report from the Star Tribune that describes some of the work being done.
Let me emphasize one point that I feel very strongly about: no one takes chemotherapy for the fun of it. If you have cancer, and your disease is at a stage when you need treatment, GO FOR IT!!! Don't let worries about side effects make you lose sight of the main goal, controlling the malignancy, living to fight another day. I get a lot of emails from people who want to avoid chemotherapy at all costs, while they wait for a breakthrough that will magically cure them, with no down-sides. I have a name for this syndrome: keeping yourself "pure for the cure".
I hate to break it to you nice people, until and unless a real cure is found, chemotherapy, radiation and passive immunotherapy such as monoclonals (read Rituxan and Campath) are it, there is not much else out there for CLL right now. The situation may change, hopefully sooner rather than later. But until that happens, if your situation becomes such that some treatment is needed and necessary, you go and find yourself the best doctor and best treatment you can find, and you go for it with everything you got. Life is a constant balance of risks and rewards. The reward of continuing to live, as opposed to the other thing, makes the risks tolerable. Which of us would refuse to live a long life, because that involves getting old? Talk about side effects, old age beats chemotherapy any day. Loss of memory, loss of physical strength, sexual dysfunction, you name it, old age has it all. Yet Granny and Grandpa seem quite happy to be alive.
We all find reserves of courage when faced with adversity. May you find strength you need to make the decisions you must, when and if the time comes for it.
The following links provide some research-based evidence of the existence of this effect, drawing their conclusions from the same Canadian study on breast cancer chemotherapy patients.
WebMD article by Paula Moyer.
Memory, Concentration Problems May Be Related to Chemotherapy
http://www.webmd.com/content/article/24/1662_50383
________
The following comes from the breast cancer action group. Be aware that the patients the report talks about are therefore breast cancer patients, with different chemotherapy drugs. But the study is nevertheless compelling reading.
"Many of us who have undergone chemotherapy have noticed changes that we were not informed about and are not acknowledged when we bring them up to our physicians. There are hopeful signs that two of the most distressing persistent effects—changes in the brain function and in sexual function—are surfacing as subjects worthy of serious study.
Persistent Effects of Chemotherapy on the Brain
Mary H. Wieneke, Ph.D., recently completed a research study on the persistent brain-behavior effects of adjuvant chemotherapy in breast cancer patients. Wieneke has written, "Cancer patients' complaints of impaired cognition following chemotherapy are fairly common." Nevertheless, she notes that there have been no studies that demonstrated objective evidence of this. Her study used a comprehensive neurocognitive test battery to evaluate 28 Stage I and Stage II breast cancer patients. The subjects ranged in age from 28 to 54 and had previously completed 3 to 18 months of adjuvant chemotherapy.
Her findings are disturbing.
Based on estimated pre-treatment I.Q.'s (these were higher than average, with a mean I.Q. of 113), over one-third of subjects showed an 11% or greater deterioration in overall I.Q. Subjects scored significantly below test norms in several areas. The affected areas were verbal and visual memory, mental flexibility and speed of processing, motor function, and visuospatial ability. 75% of patients scored in the moderately impaired range on one or more test measures. The level of impairment was not related to extent of depression, type of chemotherapy, or time since treatment. Only the length of the chemotherapy treatment (presumably meaning the amount of chemotherapy) was related to the impairment. Interestingly, except for subjective complaints of depression, patient complaints were statistically unrelated to objective test findings."

Date: 5/14/02
by Chaya Venkat
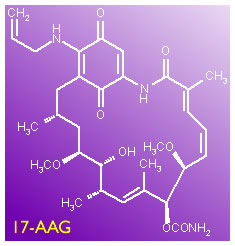 Here is a brief (and I mean brief!) overview of how 17AAG is supposed to work:
Here is a brief (and I mean brief!) overview of how 17AAG is supposed to work:
"p53" is an extremely important tumor suppressor gene. Its inactivation, for whatever reason but primarily due to mutation or deletion, is the seminal event that seems to trigger the onset of many types of cancers. It is interesting that while different human cancers exhibit very distinct profiles, perturbation of the p53 pathway occurs in most if not all of the cancers studied. p53's main role seems to be to induce apoptosis or cause growth arrest in permanently damaged cells which would otherwise trigger onset of cancer. If this cell-cycle checkpoint pathway is not working properly, the damaged cells are allowed to replicate and proliferate.
The protein expressed by p53, like other proteins, is a very complex and large molecule that has a specified three dimensional shape for it to work correctly. The exact folding of this very large protein into the correct shape is crucial to its function. Heat shock proteins (HSPs) are so-called molecular chaperones that work as protein folding machines. It has recently been identified that several HSPs, such as HSP40, HSP70, HSP90 etc are involved in the inactivation of p53 by altering its folding pattern from the correct one. Furthermore, the enhanced stability of the HSP-mutated p53 complex means it will accumulate to high levels within the body.
17AAG (17-allylamino glendanamycin) has been found to block the effect of HSP90. This is thought to prevent the mutation of p53, without causing any other DNA changes. In that sense this approach is considered to cause no damage to the DNA, unlike standard chemotherapy drugs. Since p53 deactivation is primary to most cancers, this approach is hoped to have wide applicability. However, I do not understand how it would be of use in cases where the cancer is due to deletion of the p53 gene.
The first clinical trial of 17AAG is being conducted at the Royal Marsden Hospital, UK.
Biochem Journal Article: Strategies for manipulating the p53 pathway in the treatment of human cancer - More about p53 and heat shock proteins, etc.
The subject of p53 tumor suppressor gene (see Meet the Tumor Suppressor) is an extremely important one, since it has implications for a variety of therapeutic approaches. For example, people who have p53 deletion or defect are supposed to be less responsive to drug treatment, or anti-angiogenesis control of their tumors. As usual, most of the information available to date deals with "solid" tumors, not blood cancers. Lots and lots of arms and legs to this one.
For more on 17AAG and heat shock proteins, read our 2026 articles on the subject: 17AAG and 17AAG Clinical Trial.

Cyclophosphamide (Cytoxan)
Date: 4/28/02
by Chaya Venkat
 This is another of my 'cheat-sheets' on frequently used chemotherapy drugs in the treatment of CLL. The usual disclaimer is in effect; this information is for the purpose of facilitating your discussions with your doctor, and not to be taken as medical advice.
This is another of my 'cheat-sheets' on frequently used chemotherapy drugs in the treatment of CLL. The usual disclaimer is in effect; this information is for the purpose of facilitating your discussions with your doctor, and not to be taken as medical advice.
CancerBACUP : Cyclophosphamide (Endoxana®) - A user-friendly British version of the fact sheet on Cyclophosphamide.
NLM: http://www.nlm.nih.gov/medlineplus/druginfo/medmaster/a682080.html - This is the official take on Cyclophosphamide from the National Library of Medicine.
WebMD: Drugs and Treatment: Cyclophosphamide IV - This is the usual WebMD write-up, some useful information but a lot of boilerplate to get through.
RXList: http://www.rxlist.com/cgi/generic/cyclophosphamide_pi.htm - This site provides links to many other sites that you may wish to visit.

Chlorambucil (Leukeran)
Date: 4/28/02
by Chaya Venkat
 This is the old standard therapy for CLL. It is still in wide use elsewhere, but it is my impression that it has now been replaced by other more recent drugs in the USA. It is generally marketed as brown 2mg tablets and yellow 5mg tablets. One of the major advantages of this drug is that it s administered orally, and unlike intravenous injections or infusions, the patient can administer it at home without needing daily visits to the doctor's office. It is recommended that you drink a lot of water while taking this medication.
This is the old standard therapy for CLL. It is still in wide use elsewhere, but it is my impression that it has now been replaced by other more recent drugs in the USA. It is generally marketed as brown 2mg tablets and yellow 5mg tablets. One of the major advantages of this drug is that it s administered orally, and unlike intravenous injections or infusions, the patient can administer it at home without needing daily visits to the doctor's office. It is recommended that you drink a lot of water while taking this medication.
Here is the quick view of what it does, and what to expect:
CancerBACUP: Chlorambucil (Leukeran) - This British site gives you some user-friendly information about the drug.
ACOR: http://www.acor.org/leukemia/surv6.html - This site has some of the statistics of response rates, remissions, etc for Chlorambucil and the other chemotherapy drugs. Note that the sample sizes are not very large, so the statistics may be somewhat sketchy. I also do not know when this data-base was last updated.
Leukeran Website: http://us.gsk.com/products/assets/us_leukeran.pdf - This is the manufacturer's information site. It has a lot of material that you can read, some amount of legal "Cover Your A$$" type of stuff to wade through, but that is unavoidable in today's world.

Results Do Vary
Date: 4/28/02
by Chaya Venkat
We are all unique individuals and we react to chemotherapy in individual ways. Some of us experience very minimal side effects, while others of us may have a tough time with a particular drug. The same thing goes for the efficacy of a specific chemotherapy protocol. We are just beginning to learn how to tell ahead of time when a particular chemo-drug is likely to work for a specific patient, and the right treatment protocol to use, ahead of actually trying it out. But that is the topic of another article. Most people have regularly scheduled visits with the oncologist while on chemotherapy, so that the effect of the treatment can be monitored. That is the time to discuss with him/her how it is working for you, and any side effects that you may be experiencing. I thought it might be of use to review many of the drugs currently used for treating CLL. I hope these cheat-sheets will help you to discuss the issues more thoroughly with your doctor. Please bear in mind these articles are for general discussion purposes only, and the only real medical advice that you can act on is what you get from your doctor/oncologist. You will also find links to online datasheets on chemotherapeutic agents in the Reference section under Cancer Drug Manuals.
 Enter Keywords: |
———
Disclaimer: The content of this website is intended for information only and is NOT meant to be medical advice. Please be sure to consult and follow the advice of your doctors on all medical matters.
Copyright Notice:
Copyright © 2026-2007 CLL Topics, Inc. All Rights Reserved.
All materials contained on this site are protected by United States copyright law and may not be reproduced, distributed, transmitted, displayed, published or broadcast without the prior written permission of CLL Topics, Inc. You may not alter or remove any trademark, copyright or other notice from copies of the content.
However, you may download and print material from CLLTopics.org exclusively for your personal, noncommercial use.
———
