 |
||||||||||
Date: August 12, 2026
by Chaya Venkat
Index Page for Related Articles:
HuMax-CD20
HuMax-CD20 is going to be an important drug for CLL patients – I have little doubt about it.
I expect that the initial FDA approval may be for refractory patients, based on the results of Genmab’s recently announced Phase-3 trial for fludarabine and Campath refractory patients. But I am willing to bet dollars to donuts that as soon as the drug is available commercially, any number of physicians and their patients will be looking at using this new drug in a bewildering array of off-label scenarios, both as frontline / single agent therapy and in combination with other drugs. “Ofatumumab” is not a name that rolls easily off of your tongue and we will therefore continue to use “HuMax-CD20” as the handle in our articles. Folks, it is time for you to get real familiar with the ins and outs of this drug. Chances are excellent that you will encounter this drug at some point in your CLL journey, providing your insurance company and national healthcare systems cooperate, of course. Strictly as an anecdote, the response that our hypothetical hero “Harvey” has had to this drug has far exceeded my fondest hopes, and compared to Rituxan has produced a far better response with no adverse effects thus far (barring the short lived urticaria or rash during the very first infusion: see Harvey Is Back). I am truly rooting for rapid FDA approval of this drug for our patient community.
CLL Topics has been fortunate in developing excellent contacts with many of the top researchers and scientists in the CLL community. These generous folks are our unofficial advisory board and their feedback is of great importance in keeping our articles grounded in good science. Our recent article on how HuMax-CD20 works (A Smarter Monoclonal on Trial) was admittedly a “cartoon” version, but rest assured we checked our facts with several heavy-weight experts before we published our review. Our ongoing dialogue with our advisory board has raised an additional issue worth noting, one that falls into the category of potential risk. If you are planning to participate in the new Genmab HuMax-CD20 Phase-3 trial, it is my considered opinion that you read, understand and act upon the information presented in this article. This is your heads-up, folks.
As we have discussed before, complement is the name given to an interlinked cascade of proteins that is very crucial in protecting us from infections. Did you know complement is one of the most primitive forms of self defense invented by multicellular organisms, long before more sophisticated immune functions such as B-cells, immunoglobulins and T-cells were invented? Even to this day, many small organisms that cannot afford the complex “smart” immune system functions such as T-cells and B-cells have complement systems that are almost identical to the one you and I have. Millions of years of evolution went into this very robust, quick-acting defense system.
The major strength of complement is that it goes to work almost immediately, within minutes of an infection. When your defenses have been breached and the invading pathogen has gained access to your body, the first and overwhelming response comes from complement. Our bodies are hard-wired to recognize many of the common pathogens that we are exposed to, day in and day out, and when one of them gains access to our insides, the complement protein cascade becomes mobilized instantly. There is no time lost in dithering, no time wasted in getting troops ready for battle, no double checks to make sure of the exact identity of the bad guy, no forms to fill out in triplicate before weapons are issued to the fighting troops. Right away the dangerous invader is coated with millions of particles of complement proteins, and most often this is enough to kill it dead on the spot.
As we said above, complement can kill all by itself. If the right immunoglobulins are around, great, they can pitch in too. The same goes for neutrophils, macrophages and the rest of the effector cells. But generally this more concerted co-operation between complement, immunoglobulins and effector cells takes a bit longer to gear up and get ready for battle. The last (but very important) defense our bodies mount is from T-cells. Once they are told about the invasion by antigen presenting cells such as dendritic cells, and thereby recognize the invader as a legitimate target, huge armies of killer T-cells are grown to attack the specific invader. This powerful and very sophisticated process can take more than a couple of days to gear up. Think of T-cell defenses as long term and sophisticated immune protection, a bit like bringing in the big guns of the federal troops: smart, powerful, but slow to get mobilized and often handicapped by redtape and regulations. Complement is more like the underpaid cop on the beat, perhaps not as smart as the highly specialized federal SEALS, but some one who knows all the neighborhood thugs that lurk in the alleys and more often than not the cop can respond right away without missing a beat when he sees a crime happening. Below is a diagram that gives you a sense of the time scales involved in each of these defense mechanisms used by our bodies.
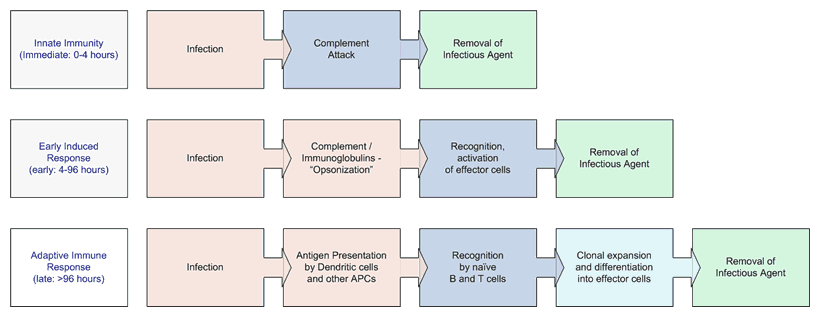
The three phases of response to an initial infection
Diagram adapted from Immunobiology 5, Janeway, et al.
What makes complement so important is its immediate and overwhelming response as soon as an infection is detected. Most of the time, you will not even know you had been invaded, because your complement system took care of the bugs well before you noticed anything, nipped the incipient infection in the bud before it had a chance to grow. Full-fledged symptoms only develop as a response to a growing infection. By the time you notice the headache, hacking cough, fever, drippy sinuses, diarrhea, sores and the like, the bug has had a chance to multiply into very large colonies. Now it is necessary to bring in the big guns of the immune system, and you may even need a little extra help in the form of a trip to the doctor — to get a prescription for man-made antibiotics or other drugs.
Complement can play a very important role in therapy using monoclonal drugs such as Rituxan, Campath and the new kid on the block, HuMax-CD20. We compared the differences in the way Rituxan and HuMax-CD20 use this very important system in our previous article (A Smarter Monoclonal on Trial). It appears complement is much more effectively used by HuMax-CD20 than Rituxan, and this can be the single most important reason why HuMax-CD20 may prove to be better suited for CLL patients.
That brings us to the crux of the issue I would like to discuss today. Complement gets used in monoclonal therapy. It gets used-up in monoclonal therapy. Below is the abstract of an elegant and important paper that looked at complement levels as a patient went through weekly Rituxan infusions. You can also read the full text for free by clicking on the link provided below.
Article from Journal of Immunology
J Immunol. 2026 Mar 1;172(5):3280-8.
Rituximab infusion promotes rapid complement depletion and acute CD20 loss in chronic lymphocytic leukemia.
Kennedy AD, Beum PV, Solga MD, DiLillo DJ, Lindorfer MA, Hess CE, Densmore JJ, Williams ME, Taylor RP.
Department of Biochemistry and Molecular Genetics, University of Virginia School of Medicine, Charlottesville, VA
Complement plays an important role in the immunotherapeutic action of the anti-CD20 mAb rituximab, and therefore we investigated whether complement might be the limiting factor in rituximab therapy. Our in vitro studies indicate that at high cell densities, binding of rituximab to human CD20(+) cells leads to loss of complement activity and consumption of component C2. Infusion of rituximab in chronic lymphocytic leukemia patients also depletes complement; sera of treated patients have reduced capacity to C3b opsonize and kill CD20(+) cells unless supplemented with normal serum or component C2. Initiation of rituximab infusion in chronic lymphocytic leukemia patients leads to rapid clearance of CD20(+) cells. However, substantial numbers of B cells, with significantly reduced levels of CD20, return to the bloodstream immediately after rituximab infusion. In addition, a mAb specific for the Fc region of rituximab does not bind to these recirculating cells, suggesting that the rituximab-opsonized cells were temporarily sequestered by the mononuclear phagocytic system, and then released back into the circulation after the rituximab-CD20 complexes were removed by phagocytic cells. Western blots provide additional evidence for this escape mechanism that appears to occur as a consequence of CD20 loss. Treatment paradigms to prevent this escape, such as use of engineered or alternative anti-CD20 mAbs, may allow for more effective immunotherapy of chronic lymphocytic leukemia.
PMID: 14978136
____________
The diagram below comes from the paper cited above. As you can see, with each weekly Rituxan infusion, complement levels take a big hit. The good news is that complement levels do recover, over time. You can see that in the diagram, complement levels struggle to get back up between each weekly Rituxan “hit”, but never quite make it all the way to the levels at the start of the Rituxan therapy. When there is a longer gap between the first four weeks of Rituxan and the second set of 4 infusions, complement levels make it almost all the way to pre-treatment levels. The moral of the story — complement gets used up in Rituxan therapy and it takes time for this important immune system function to recover.
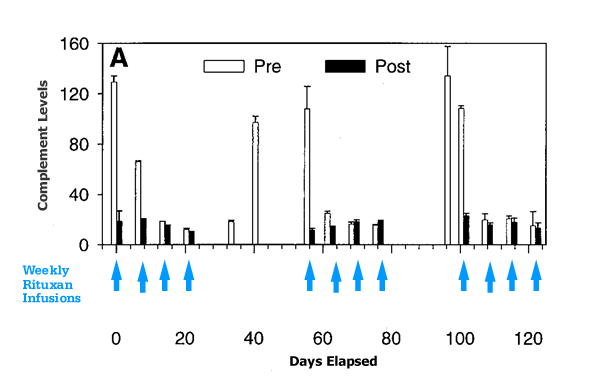
Complement Depletion in Rituxan Therapy
Diagram adapted from J. Immunology, Kennedy, et al.
I have often wondered about the logic and advisability of single-agent Rituxan protocols that use mega doses of Rituxan, or specify thrice weekly infusion schedules. If complement recovery cannot handle even weekly infusions of Rituxan at the “standard” dose of 375 mg/m2, it stands to reason that more frequent infusions or larger doses each week will only serve to drive complement levels even lower and make it harder for the levels to recover between each shot of Rituxan. But then, since Rituxan is not able to do make much use of complement mediated cancer cell kill in the first place, perhaps it is not all that important that complement is not at peak performance levels for the successive Rituxan infusions. The story is very different when it comes to HuMax-CD20, and especially in the context of the patients who will be in the new Phase-3 clinical trial using this drug.
Let us compare the HuMax-CD20 protocol that will be used in the Phase-3 trial, with the information above for Rituxan. I will be the first one to admit, this is not an apples to apples comparison, but it is the best I can do in the absence of similar data pertaining to HuMax-CD20.
I contacted Genmab, and raised this issue with them in an hour-long telephone conversation. However, I was not able to confirm whether they have looked at complement levels in prior Phase – I / II trial patients as they went through HuMax-CD20 infusions (only 4 weekly infusions in the earlier trial, as opposed to 8 weeks in this trial, followed by additional 4 monthly infusions). I did my fervent song and dance about loss of complement defenses in this group of patients who have little else to fall back on, and my concerns about potential opportunistic infections as they go through HuMax-CD20 therapy. They felt patients will be adequately protected, here is the direct quote that I am authorized to use by the company:
“Several precautions have been taken to ensure safety for the patients enrolled in the HuMax-CD20-406 trial. These include an independent Data Monitoring Committee involved in ongoing close review of safety data, and an Internal Safety Committee for regular surveillance of safety data.”
I don’t know about you, but this was not enough to set my heart at ease. I pushed further. I pointed out that use of prophylactic broad-spectrum antibiotics and antiviral drugs is now mandatory in Campath therapy, to protect patients against opportunistic infections. I like to be safe rather than sorry. I guess I am a belt-and-suspenders-and-cummerbund type of person, and not ashamed of it. If something can be done ahead of time that prevents infections in our patients, rather than dealing with them after the fact, I am all for it. Remember folks, the majority of deaths in CLL patients is due to infections! (Infectious Complications in CLL).
It is next to impossible to change clinical trial protocols at this late stage, I am sympathetic and fully aware of that. To be fair, Genmab did not think there was an issue here, they did not think the patients are at increased risk of opportunistic infections and therefore they did not see a need of pre-emptive medications. They did not feel the complement depletion in Rituxan therapy can be extrapolated to HuMax-CD20 therapy. One patient did die of pneumonia in their earlier trial, but this patient had a history of prior hospitalizations due to pneumonia (See document titled "Significant Correlation between Objective Response and Exposure to HuMax-CD-20 in CLL"). Being the worry-wart that I am, I wondered if even this patient, with documented susceptibility to pneumonia going into the trial, may have fared better if his complement levels had not been depleted. But that is water under the bridge, and there is not much point worrying about it now.
We went round and round this mulberry bush for about an hour. Finally we came up with a pragmatic consensus that I am happy to report. If patients going into this trial feel they may be at risk of opportunistic infections, especially bacterial and respiratory tract infections, or if they have had prior run-ins with pneumonia and the like, they are at liberty to discuss these concerns with their own oncologists or general practitioners ahead of time and get on prophylactic medications before starting HuMax-CD20 therapy. Use of prophylactic medications (broad spectrum antibiotics and antiviral drugs) will not trigger exclusion from this clinical trial. In other words, if this issue worries you and your doctor, you can take care of it yourself.
That agreement from Genmab satisfied me. If you are a participant in this clinical trial, I cannot tell you that you must take these precautions before starting on the HuMax-CD20 trial. That would be practicing medicine without a license, and I am merely an interested layperson reporter. But I can tell you that if you have concerns along these lines, especially if you have had to deal with respiratory tract infections (or other chronic infections) in the past, I think it is a darn good idea to talk it over with your GP.
Today we have a wide variety of broad spectrum antibiotics available to us. Some of the names of these drugs are almost familiar household names, at least in the CLL community. You may have had to use some of them already. You and your GP know which ones work well for you. While it is not a good idea to over-indulge in broad spectrum antibiotics (none of them are entirely without side effects, and their inappropriate use can lead to creation of resistant “super bugs”), this may be an occasion when their use is entirely justified. For crying out loud, CLL patients are encouraged to be on protective broad-spectrum antibiotics before they get dental work done! Surely participating in this clinical trial is at least as important as getting your pearly whites cleaned! If you write and tell me that you cannot negotiate getting a script from your GP for Bactrim, Z-pack or whatever works well for you as you go into this clinical trial, fie on you. You need a course in being a bit more assertive in the gentle art of negotiation.
That’s it, folks. I have done my job as your devoted patient advocate, and I have touched base with our unofficial advisory board of experts on this issue to make sure I am not off-base here. Now it is your job to read, understand, and act upon the information we have presented here. Be smart, be well.
 Enter Keywords: |
———
Disclaimer: The content of this website is intended for information only and is NOT meant to be medical advice. Please be sure to consult and follow the advice of your doctors on all medical matters.
Copyright Notice:
Copyright © 2026-2007 CLL Topics, Inc. All Rights Reserved.
All materials contained on this site are protected by United States copyright law and may not be reproduced, distributed, transmitted, displayed, published or broadcast without the prior written permission of CLL Topics, Inc. You may not alter or remove any trademark, copyright or other notice from copies of the content.
However, you may download and print material from CLLTopics.org exclusively for your personal, noncommercial use.
———
