 |
||||||||||
Date: October 14, 2026
by Chaya Venkat
Related Articles:

All too often, the emphasis of research and clinical trials is focused on newly diagnosed or “naïve” ( = previously untreated) patients. But the reality is that except for a minority of exceptionally lucky CLL patients with truly smoldering disease that never needs treatment, for most of us the disease will progress over time and will need therapy to control it. This article is all about the choices and risks faced by patients who have been around the CLL therapy track more than a few times, old timers that have already indulged in their fair share of chemotherapy drugs. If you are the type that would rather know what you are facing straight up, read on. Frankly, I do not see how one can make smart therapy choices without knowing the risks, rewards and choices. But if you are feeling a tad vulnerable right now, it is not a bad idea to bookmark this article for reading at a later time. Don’t say I did not warn you!
Whether it is single agent monoclonal antibodies (like Rituxan, Campath, or Humax-CD20), or single agent chemotherapy (like chlorambucil, fludarabine), or more sophisticated combination of monoclonals and chemotherapy drugs (like RF, RFC, CFAR), there is little doubt that the very process of going through one or more layers of therapy changes the nature of the CLL clone. With each round of therapy, the cancer cells learn a few more tricks of how to avoid getting killed, how to survive and grow.
A lot has been written about drug resistance and how it develops (see Multi-drug Resistance to Chemotherapy). For simplicity, let us consider therapy with single agent fludarabine. (Generally similar patterns are responsible for drug resistance with other drugs.) Not that long ago, fludarabine was the unofficial “gold standard frontline therapy” and most patients got treated with this purine analog as the first choice when they got done with the ever popular “Watch & Worry”. With the advent of modern prognostics and better ability to distinguish between genetic abnormalities, this is not quite the slam dunk choice any more. For example, patients who have the dreaded 17p (p53) deletion as determined by FISH test would be ill advised to go this route. Now we know that fludarabine needs the p53 pathway to do its CLL cell killing. If your CLL cells are deficient in this very important gene and the cell kill pathway it controls, then fludarabine will not do you much good.
What happens if you have a mixed population of CLL cells? What if you have 99% of your CLL cells with the more benign 13q deletion, and a tiny percentage, no more than 1% of your CLL cells, have just picked up a new abnormality, the 17p deletion? Clonal evolution is a fact of life and there is a good chance that over time your CLL will evolve to pick up more abnormalities. If this is the scenario, a FISH test would not even detect the 17p deletion: the new “baby clone” would be too small and the signal too faint to be picked up by FISH test. With no obvious reason not to use fludarabine therapy, it might well be used as the frontline choice. And the results will look great, at first. The patient will be delighted to see close to 99% reduction in his blood counts, his lymph nodes will shrink dramatically and he will be declared to be in “complete response”, a gold plated “CR” success story.
What has actually happened here? Most, if not all of the cells with the 13q deletion as the sole abnormality are only too easily killed by fludarabine and this corresponds to the rosy picture the patient sees immediately at the completion of the therapy. But that is not the whole story. A nagging little bit of the CLL is left over, the 1% of the total CLL population that also had the 17p deletion, since this bunch cannot be killed by fludarabine. There is now a new boss in town, the baby clone is now in charge — with no competition for resources or space, nothing to keep it under check. Chances are good it will grow and flourish quickly, gradually taking over all the eco-niches that used to be occupied by its larger sister clone. When the patient's CLL relapses, and it is only a question of when and not if that the disease will relapse, the prognosis is a lot darker. Now the majority of this patient's CLL cells cannot be killed by fludarabine. If he is careless enough to try fludarabine once more, without first checking his FISH status, he will get very little for his efforts – all that hassle and toxicity of chemo and not much to show for it. He is now formally classified as “fludarabine refractory” – a scary phrase, as we will see further down in this review.
Incidentally, in all of this explanation we did not even invoke the mutagenic nature of fludarabine, its ability to create brand new genetic abnormalities where none existed before. Just the simple process of tough-to-kill cells surviving and thriving is enough to explain gradual drug resistance: natural selection. Plain old Rituxan therapy can do the same thing, gradually losing its zing with multiple cycles of use. Did you know that even if you had been treated with nothing but Rituxan, your chances of responding to FCR therapy are significantly less than if you had never been treated at all, an entirely treatment-naïve patient?
The abstract below from the NCI emphasizes the same points. You can read the full text of the article by clicking on the link. The second abstract highlights the dangers faced by fludarabine refractory patients.
Full Text Article from Blood Journal
Blood. 2026 Sep 1;104(5):1428-34. Epub 2026 May 11.
Fludarabine treatment of patients with chronic lymphocytic leukemia induces a p53-dependent gene expression response.
Rosenwald A, Chuang EY, Davis RE, Wiestner A, Alizadeh AA, Arthur DC, Mitchell JB, Marti GE, Fowler DH, Wilson WH, Staudt LM.
Metabolism Branch, Center for Cancer Research, National Cancer Institute/NIH, Bethesda, MD.
Fludarabine, the current standard treatment for B-cell chronic lymphocytic leukemia (CLL), can induce apoptosis in CLL cells in vitro, and a number of molecular mechanisms contribute to its cytotoxicity. Using gene expression profiling, we investigated the molecular consequences of fludarabine treatment of patients with CLL in vivo. In 7 patients with CLL, a consistent gene expression signature of in vivo fludarabine exposure was identified. Many of the fludarabine signature genes were known p53 target genes and genes involved in DNA repair. In vitro treatment of CLL cells with fludarabine induced the same set of genes as observed in vivo, and many of these genes were also induced by in vitro exposure of CLL cells to ionizing radiation. Using isogenic p53 wild-type and null lymphoblastoid cell lines, we confirmed that many of the fludarabine signature genes were also p53 target genes. Because in vivo treatment with fludarabine induces a p53-dependent gene expression response, fludarabine treatment has the potential to select p53-mutant CLL cells, which are more drug resistant and associated with an aggressive clinical course. These considerations suggest that fludarabine treatment should be given in strict accordance to the current National Cancer Institute (NCI) guidelines that have established criteria of disease activity that warrant treatment.
PMID: 15138159
____________
Cancer. 2026 Apr 1;94(7):2033-9.
Frequency and type of serious infections in fludarabine-refractory B-cell chronic lymphocytic leukemia and small lymphocytic lymphoma: implications for clinical trials in this patient population.
Perkins JG, Flynn JM, Howard RS, Byrd JC.
Department of Medicine, Walter Reed Army Medical Center, Washington, D.C.
BACKGROUND: Treatments for fludarabine-refractory chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL) are limited. Most new therapies being examined in fludarabine-refractory patients have shown a high frequency of serious infection. Little data exist regarding the frequency of infections in this population treated with noninvestigational best supportive care therapies.
M
ETHODS: The infectious courses of 27 patients with fludarabine-refractory CLL/SLL were retrospectively reviewed. Fludarabine-refractoriness was defined as either relapse within six months of completion of or failure to respond to fludarabine treatment. Infections were documented after patients met National Cancer Institute criteria for further treatment. Serious infections were defined as infections mandating admission to the hospital for intravenous antibiotics.
RESULTS: Patient characteristics included: median age 67 years (range, 40-83), median 3 chemotherapy treatments (range, 1-8), and hypogammaglobulinemia in 73% of patients. Pneumocystis carinii prophylaxis was given to 89% of patients. Serious infections developed in 24 out of 27 patients (89%). Patients had a median of 2 admissions (range, 0-11) for serious infection occurring at a median of 4 months (range, 0-21) from onset of fludarabine-refractoriness. The median frequency of admission for infection was 0.17 per month. Most common sites for infection in decreasing frequency were: respiratory tract, urinary tract, blood/sepsis, and soft tissues. Bacteria caused 69 out of 88 infections (78.4%); viruses (varicella-zoster and herpes simplex) caused 11 out of 88 (12.5%); fungi caused 4 out of 88 (4.5%); and opportunistic infections caused 4 out of 88 (4.5%). Median survival was 13.0 months (range, 1-44+).
CONCLUSIONS: The frequency of serious infections in patients with fludarabine-refractory CLL/SLL is high. These findings are relevant to trials with new and highly effective agents for which the incidence of serious infections after treatment might otherwise appear to be prohibitively high. Copyright 2026 American Cancer Society.
PMID: 11932906
____________
Dr. Montserrat is one of the grand old men of CLL, right up there with Dr. Rai, et al. In a very clearly written 2026 article, Dr. Montserrat distilled his decades of expertise into a few pages. The abstract is below, as well as the link to the full text of the article.
Full Text Article from Blood Journal
Blood. 2026 Feb 15;107(4):1276-83. Epub 2026 Oct 4.
How I treat refractory CLL.
Montserrat E, Moreno C, Esteve J, Urbano-Ispizua A, Giné E, Bosch F.
Institute of Hematology and Oncology, Department of Hematology, Hospital Clínic, Villarroel, Barcelona, Spain.
Therapy for patients with chronic lymphocytic leukemia (CLL) has greatly changed over the past few years. After years of stagnation, with treatment revolving around the use of rather ineffective drugs such as alkylators, many patients are now being treated with more effective agents such as purine analogs either alone or combined with other drugs and/or monoclonal antibodies. Treatment of patients refractory to these treatments is particularly challenging and should be decided only upon a careful evaluation of the disease, patient characteristics, and prognostic factors. Refractory disease should be clearly separated from relapsing disease. The only curative therapy for patients with CLL, including those with refractory disease, is allogeneic stem cell transplantation. However, the use of allogeneic transplantation is limited because of the advanced age of most patients and the high transplant-related mortality (TRM). Transplants with nonmyeloablative regimens may reduce TRM and allow more patients to receive transplants more safely. For patients in whom an allogeneic transplantation is not feasible or in whom it is deemed inappropriate, participation in phase 2 trials should be encouraged. Finally, to investigate mechanisms to overcome resistance to therapy in CLL and to identify patients that might gain benefit from early, intensive therapies (eg, based on biologic markers) constitute a challenge that needs active investigation.
PMID: 16204307
____________
Dr. Montserrat point out there is no formally accepted definition for refractory CLL, but a reasonable definition of refractory CLL is either the lack of response to purine analogs (fludarabine or pentostatin) or disease progression within 6 months of getting a PR or CR. The prognosis of patients with refractory CLL is very poor, their median survival being measured in months. (Editors Note: perhaps somewhat longer now, with the advent of Campath and possibly flavopiridol).
The author sounds a very important caution: when confronting a patient with refractory CLL, it is important to make sure that the patient actually has CLL and not another disorder that mimics CLL. Sometimes, patients deemed to have refractory CLL turn out to have another disease altogether. For example, mantle-cell lymphoma is sometimes mistaken for refractory CLL (A Wolf in CLL Clothing). Another important point is to rule out the CLL has morphed into a more dangerous and different beast altogether. In about 10% of patients, CLL undergoes transformation into diffuse large B-cell lymphoma (DLBCL), also known as Richter’s transformation. CLL can also transform into Hodgkins lymphoma. Both of these transformations are usually associated with prior chemotherapy and/or EBV (Epstein-Barr virus). Since mantle cell lymphoma, Richter’s syndrome and Hodgkin lymphoma are a lot more aggressive and requite very different treatment strategies, you can see why it is important to sort all this out before jumping to conclusions.
Campath has become an important drug in CLL. Its major advantage is that it does not seem to be stymied by lack of 17p (p53) expression. But it is important to remember that Campath is more effective in peripheral blood, less so at other locations. Large lymph nodes are particularly resistant to Campath, the presence of lymph nodes larger than 5 cm being correlated with poor response. Getting a CT scan done ahead of embarking on Campath therapy may be useful to predict response. Campath is not exactly an easy drug to tolerate, well known for its deep immune suppression and T-cell destruction. Almost all patients are at risk of deep neutropenia. Unless they are treated with prophylactic medications, there is serious risk of viral infections, both of the opportunistic variety as well as reactivation of lurking traces within them. If your lymph nodes at the time of becoming officially fludarabine refractory are bigger than 5 cm, you are, for all practical purposes, Campath refractory as well.
As you can imagine, finding drugs that work in patients with p53 defects is the holy grail of CLL research. Even if you have a fully functional p53 today, there is no guarantee that down the road your CLL will not acquire this dangerous abnormality down the road, either as a result natural clonal evolution or because of chemotherapy. Think of it this way. When you are young and have a full head of hair, you can afford to snigger at all the older guys standing in front of the “Rogain” display at the drugstore. But most young studs grow up to become balding old coots, with only fading memories of bygone glory. Moral of the story, it is in all of our interest to learn about drugs that may work for these tough cases. You may well have to walk in their shoes down the road. I am a little disappointed the list is so short: Campath (aka alemtuzumab), high dose steroids and possibly flavopiridol. That’s it! The same cast of characters we have been discussing for several years now.
Best Pract Res Clin Haematol. 2026 Sep;20(3):545-56.
Novel agents and strategies for treatment of p53-defective chronic lymphocytic leukemia.
Grever MR, Lucas DM, Johnson AJ, Byrd JC.
Division of Hematology-Oncology, Department of Internal Medicine, The Ohio State University, Columbus, OH, USA.
Chronic lymphocytic leukemia (CLL) is a common leukemia with a highly variable natural history. A subset of patients with high-risk CLL rapidly progress to develop symptomatic disease requiring treatment. Over-represented in this group are those who have a deletion of 17p13.1, the chromosomal location of the tumor suppressor gene P53. Of all prognostic factors examined in CLL, del(17p13.1) has a superior predictive value for poor response to conventional therapy. In this article we review the current published data on prognostic factors relevant to treatment in CLL. We next provide therapeutic recommendations for patients with del(17p13.1) that are available to oncologists in general practice. Chemoimmunotherapy, alemtuzumab, or high-dose corticosteroids are all effective as initial therapy for these patients, but progression is generally rapid. If allogeneic immune therapy is to be considered, it should be approached as part of initial or first salvage therapy. The investigational agent flavopiridol has also demonstrated clinical activity in this subset of patients. Identification of small molecules and new treatment approaches for patients with del(17p13.1) is a major focus of several investigators. Selection of therapy based on high-risk genomic features represents an appropriate treatment approach supported by currently available published data.
PMID: 17707839
_____________
Does Campath do a bang up job on all fludarabine refractory patients, with the only caveat that they must not have lymph nodes larger than 5 cm? Not quite. The table below is from a Berlex publication. This is what the company has to say about Campath responses in fludarabine refractory patients. It is definitely a half-full glass. Pretty stiff price of toxicity and morbidity, and only a measly 4% of patients got a “CR”. But to be fair, I get the sense that we are learning more about how to administer this drug, what precautions to take, how to control infections risk and so on, giving me hope that current experience will be actually quite a bit better than these reported statistics. Especially in combination with high dose steroids (see Steroid-Campath Combinations), Campath is a powerful and valuable drug for refractory patients.
The table below is taken from CLL: The Cutting Edge, a Berlex publication on Campath in fludarabine refractory CLL, authored by Dr. William G. Wierda of the M. D. Anderson Cancer Center.
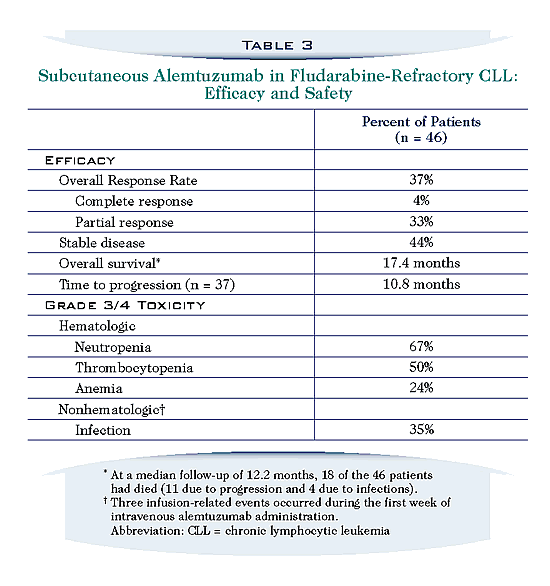
Taking into consideration all of these points as well as the patient’s age, general level of fitness, co-morbidities, etc, Dr. Montserrat proposes mini-allo stem cell transplantation as one of the better choices for patients who are fludarabine and Campath refractory. But sometimes this is easier said than done. First and foremost, the patient must have a well matched stem cell donor, either related or unrelated. In this regard, Dr. Montserrat points out a potential risk: it has recently been demonstrated that up to 13.5% of first-degree relatives of patients with may themselves harbor the tell-tale CLL phenotype (Familial CLL; Not the Worst Day of Your Life). You would hardly want to go through all the pain and suffering of a mini-allo transplant to get rid of your CLL once and forever, only to replace it with some one else’s CLL! So this might be important to check out. I am not kidding, I have heard from three separate patients in the past year or so who had perfect sibling matches, and when it came time to harvest donor stem cells, it became clear the supposedly “healthy” donor had CLL as well! Bummer, indeed! For all parties concerned.
To Dr. Montserrat’s comments about mini-allo transplants I would like to add the fact that cord blood stem cell transplants are coming up the learning curve awfully fast. Some of the experts PC and I have been talking to suggest transplants using cord blood may be just as good if not better than adult donor stem cells. In the opinion of other experts we are not quite there yet — but it might well be the case in the future. Cord blood also has the great advantage of providing most patients with a sufficiently well-matched source of stem cells, a factor that is very important for ethnic minorities. (See: Cord Blood Transplants: the State of the Art).
Most of us know by now that flunking fludarabine and Campath does not leave too many good choices. But until I read the latest paper from M. D. Anderson (abstract below), I did not know how heavily the dice were loaded against this group of patients. If you want to read this hot-off-the-presses and tremendously important article, write to us. But be sure you are ready for some grim statistics, since this is not fun reading by any stretch of the imagination.
Leuk Lymphoma. 2026 Oct;48(10):1931-9.
The natural history of fludarabine-refractory chronic lymphocytic leukemia patients who fail alemtuzumab or have bulky lymphadenopathy.
Tam CS, O'brien S, Lerner S, Khouri I, Ferrajoli A, Faderl S, Browning M, Tsimberidou AM, Kantarjian H, Wierda WG
Department of Leukemia and Stem Cell Transplantation, MD Anderson Cancer Center.
The natural history and outcome of salvage treatment for patients with fludarabine-refractory chronic lymphocytic leukemia who are either refractory to alemtuzumab ("double-refractory") or ineligible for alemtuzumab due to bulky lymphadenopathy ("bulky fludarabine-refractory") have not been described. We present the outcomes of 99 such patients (double-refractory n = 58, bulky fludarabine-refractory n = 41) undergoing their first salvage treatment at our center. Patients received a variety of salvage regimens including monoclonal antibodies (n = 15), single-agent cytotoxic drugs (n = 14), purine analogue combination regimens (n = 21), intensive combination chemotherapy (n = 36), allogeneic stem cell transplantation (SCT; n = 4), or other therapies (n = 9). Overall response to first salvage therapy other than SCT was 23%, with no complete responses. All four patients who underwent SCT as first salvage achieved complete remission. Early death (within 8 weeks of commencing first salvage) occurred in 13% of patients, and 54% of patients experienced a major infection during therapy. Overall survival was 9 months, with hemoglobin < 11 g/dL (hazard ratio 2.3), hepatomegaly (hazard ratio 2.4), and performance status >/= 2 (hazard ratio 1.9) being significant independent predictors of inferior survival.
PMID: 17917961
____________
The Anderson "Natural Hisory" paper is pretty hard-hitting and I cannot really do it justice in a quickie cheat-sheet. With that caveat, here are some of the highlights:
The million dollar question from the perspective of patients is this: never mind what you call the remission, CR, PR, nPR or whatever, how long did the patients live after the salvage therapy? The picture below is worth a thousand words, pretty scary words at that. Not one of the groups of patients had better than 16 months median survival. By the time the second anniversary rolled around, a scant third of the patients were alive, if that.
Survival After Salvage Therapy
C. S. Tam, et al., Leukemia & Lymphoma, October 2026; 48(10): 1931 – 1939
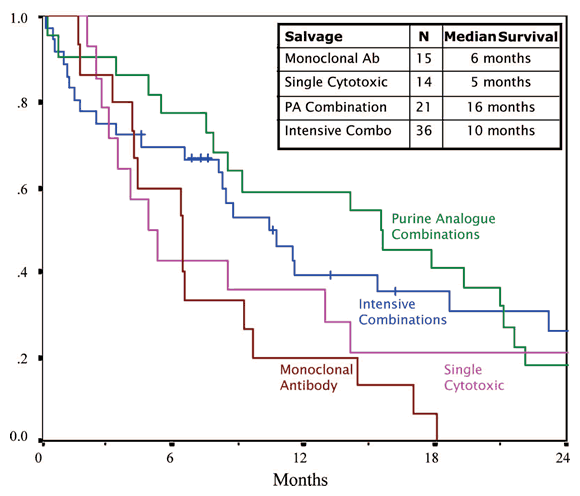
I have to admit I was taken aback by the gloomy picture painted by this important study, especially when it came to chemo-immunotherapy salvage options. With the ready availability of fludarabine and Campath (especially now, with FDA approval for frontline Campath therapy — see Jumping the Gun), the numbers of double refractory patients is going to grow much larger than in prior years. The tough news is that once patients have burned through these two lines of defense, there are not too many good salvage options left.
I doubt anyone would call refractory CLL a “good cancer” to have. Not only is the median survival shockingly short, the multiple infections and hospitalizations add up to poor quality of life. Unfortunately, except for a small percentage of very lucky patients who have truly smoldering disease that never needs to be treated, most of our guys will get to this stage, sooner or later. The title of the article is very apt: This is indeed the “Natural History of CLL”. We desperately need better options for this group of patients!
The only glimmer of hope in this article is the outcome of patients who opted to go the transplant route. Two of the 4 patients are basically cured, the CLL monkey off their back “for good”. Keep your fingers crossed. I cannot but wonder whether with improved GVHD control (GVHD, A Pebble in the Transplant Shoe) and better viral prophylaxis, even the two guys who did not make it could have been saved.
One of the mini-allo transplant experts we met at the NIH (National Institutes of Health) had interesting comments about the difference between “elective” and “salvage” transplants. He felt that if patients opted for transplants just a bit sooner, before they became truly salvage basket cases, before the co-morbidities piled on additional layers of risk, transplant statistics would show a remarkable improvement. But as we have reviewed in recent articles (Battle Plans, Catch 22) this is not an easy decision to make. Lack of sufficient health insurance, lack of well matched adult donors, advanced age and insufficient physical fitness, lack of adequate counseling or expert consensus on who is a good transplant candidate: all of these and many more obstacles stand in the way of “elective” transplants.
Reading this important paper from M. D. Anderson, our hypothetical hero “Harvey” is all the more convinced he has made the right choice in developing his battle plans. For those of you who are not already over-dosed on this depressing topic, below is an excellent CME article by no less than Dr. John Gribben. You can read the full text by clicking on the link.
Full-Text Article in ASH Education Book
Hematology Am Soc Hematol Educ Program. 2026;:292-8
Salvage Therapy for CLL and the Role of Stem Cell Transplantation.
Gribben JG.
Chronic lymphocytic leukaemia (CLL) remains an incurable disease and, notwithstanding the excellent remission rates now achieved with purine analogs and monoclonal antibodies, the vast majority of patients with CLL are destined to relapse after primary treatment. The management of relapsed CLL patients is then dependent upon a number of factors, most importantly age, performance status, previous therapy administered, the response and duration of response to such therapy, and time from last therapy. Although prior therapy and response to such therapy are important factors in determining next therapy, it is often difficult to determine their importance from published studies. Furthermore, the goal of therapy, whether palliative or aggressive, must also be weighed into the decision when deciding on the next line of treatment. With many potential treatments available, the sequence of treatments and the timing of procedures such as stem cell transplantation remain controversial and are the focus of ongoing clinical trials.
PMID: 16304394
____________
 Enter Keywords: |
———
Disclaimer: The content of this website is intended for information only and is NOT meant to be medical advice. Please be sure to consult and follow the advice of your doctors on all medical matters.
Copyright Notice:
Copyright © 2026-2007 CLL Topics, Inc. All Rights Reserved.
All materials contained on this site are protected by United States copyright law and may not be reproduced, distributed, transmitted, displayed, published or broadcast without the prior written permission of CLL Topics, Inc. You may not alter or remove any trademark, copyright or other notice from copies of the content.
However, you may download and print material from CLLTopics.org exclusively for your personal, noncommercial use.
———
