 |
||||||||||
Updated: August 11, 2026
Standard of Care
Fludarabine Monotherapy Is No Longer the Gold Standard
The Addition of Rituxan to Fludarabine Results in Longer Survival
 We review two research papers published in Blood Journal.
The first, authored by a panel of top CLL experts, presents persuasive data
that a combination of Rituxan and Fludarabine is superior to Fludarabine
monotherapy in both response rate and survival. The companion paper, authored
by researchers at the NCI, concludes that Fludarabine monotherapy in CLL relies
on a p53-dependent mechanism for cell kill and that this therapy has the potential
to select for p53-mutant cells, leading to more aggressive and resistant disease.
These are very important conclusions that need wide dissemination among patients
and practicing oncologists. Please read our article
Fludarabine Monotherapy Is No Longer the Gold Standard. (5/13/04)
We review two research papers published in Blood Journal.
The first, authored by a panel of top CLL experts, presents persuasive data
that a combination of Rituxan and Fludarabine is superior to Fludarabine
monotherapy in both response rate and survival. The companion paper, authored
by researchers at the NCI, concludes that Fludarabine monotherapy in CLL relies
on a p53-dependent mechanism for cell kill and that this therapy has the potential
to select for p53-mutant cells, leading to more aggressive and resistant disease.
These are very important conclusions that need wide dissemination among patients
and practicing oncologists. Please read our article
Fludarabine Monotherapy Is No Longer the Gold Standard. (5/13/04)
Multi Drug Resistance
Fludarabine Refractory Patients Face Fewer Choices
A Significant – and Inevitable – Obstacle
 While chemotherapy is the accepted way of dealing with the growing tumor load and other adverse effects of CLL,
it carries with it built-in limitations and dangers. One of them is the inevitable occurence of multi-drug
resistance as the cancer cells learn ways to cope with the chemotherapy attacks. Fludarabine, the most
effective chemotherapy agent in CLL, eventually fails to produce a remission in most patients and the
term fludarabine refractory makes for a grim prognosis not only because
fludarabine stops working but also because many other agents lose their effectiveness. To learn more
about this phenomenon, read our article Multi Drug Resistance
to Chemotherapy. (7/23/03)
While chemotherapy is the accepted way of dealing with the growing tumor load and other adverse effects of CLL,
it carries with it built-in limitations and dangers. One of them is the inevitable occurence of multi-drug
resistance as the cancer cells learn ways to cope with the chemotherapy attacks. Fludarabine, the most
effective chemotherapy agent in CLL, eventually fails to produce a remission in most patients and the
term fludarabine refractory makes for a grim prognosis not only because
fludarabine stops working but also because many other agents lose their effectiveness. To learn more
about this phenomenon, read our article Multi Drug Resistance
to Chemotherapy. (7/23/03)
The following are short articles on fludarabine, presented most recent first.
Dosage Is Critical
Quantities Vary Widely in the Major Combination Protocols
Date: 4/5/03
by Chaya Venkat
While it is probably desirable to be treated with only one toxic substance versus two, a member raised the point that the total amount of Fludarabine in the RF protocol in Dr. Byrd's article far exceeded that in the RFC protocol as presently practiced at MD Anderson. This is an excellent point.
I understand that the initial few patients who went through the RFC protocol at Anderson had higher dosages of Fludarabine, which was later reduced to reduce the hematological toxicity of the regimen. Our member was right to point out that the Fludarabine dosage in the Anderson version of RFC is now is lower than the Byrd RF protocol. It makes it that much harder to compare the results obtained in these two leading protocols.
This gives me one more chance to make the point, it is not enough for your oncologist to tell you that you will be getting drugs X and Y and Z. It is important to know exactly how much of these drugs you will be getting. Just prior to her therapy, one of our members asked the nurse how much Fludarabine she would be getting. The nurse answered, about 100 ml. Wow!! I said, when I heard about it. That is a LOT of fludarabine! Turns out the nurse thought the patient wanted to know how much saline was going to be infusion bag, along with a miniscule 25 milligrams of the drug. One of those failures to communicate. And to make it all the more confusing, RF and RFC come in different strengths, depending on the cancer center and the specialist one goes to. I know that the FRC protocol as it is administered in Europe is quite a bit different. Some one should do a tabulation of the different drug dosages, frequency of administration, number of cycles and pre-medications used in each of the locations, so that we can have a handy comparison chart for all of us. Any one wants to volunteer for the job?
There are new rules going into effect, supposedly to protect the privacy of patients undergoing therapy, that will make it harder for family members or “chemo buddies” to be with patients through the infusion. And there is a good chance you may not be in a frame of mind to care too much about getting down details, once the infusion has started and you are in the throes of those “side effects” that the scientific papers talk about so blithely, that are quite a bit more real when you are on the receiving end of that infusion needle. All the more important to have the details clearly understood, before you get started.
Editor's Note: You can see the dosages of various drugs in the popular chemoimmunotherapy protocols in our article Drug Dosages in Popular “Standard” Protocols.

Oral Fludarabine
As an Oral Drug, Fludarabine Gains Versatility
Date: 2/12/03
by Chaya Venkat
The mode of administration of a chemotherapy agent is an important question. It determines how much of a commitment in time and effort (and cost) is involved in a given protocol.
Fludarabine is, and will continue to be for quite a while, the leading drug for treatment of CLL, whether it is used as an stand- alone therapy or in combination with other modalities such as Rituxan etc. Until very recently, the only way to administer this important purine analogue is by means of an IV infusion, which is time consuming both for the patient and the healthcare system. Oral bioavailability means the patient can take a pill at home, and not make another trip to the doctor's office, and forgo the pleasures of another needle stick. There is another aspect of bioavailability that is not yet fully explored, and that is maintaining a constant and continuous level of the drug in the body, as opposed to the spikes one gets with weekly IV infusions.
As far as I can tell, the oral form of the drug seems to be as effective, and has no more side effects, as the IV variety. There is some indication that the oral variety may have slightly higher gastric problems associated with it, but that is probably fixed by taking the drug with food, rather than on an empty stomach. All in all, availability of the oral form in this country is a good development.
J Clin Oncol 2026 Nov 15;19(22):4252-8
Activity of oral fludarabine phosphate in previously treated chronic lymphocytic leukemia.
Boogaerts MA, Van Hoof A, Catovsky D, Kovacs M, Montillo M, Zinzani PL, Binet JL, Feremans W, Marcus R, Bosch F, Verhoef G, Klein M.
Department of Hematology, University Hospital, U.Z. Gasthuisberg, Herestraat 49, B-2000 Leuven, Belgium.
PURPOSE: A prospective, multicenter, open-label phase II clinical trial was conducted to assess the efficacy and safety of oral fludarabine phosphate. Reference to an historical group of patients treated with the intravenous (IV) formulation allowed the investigators to compare the two formulations.
PATIENTS AND METHODS: Efficacy was assessed using the International Workshop on Chronic Lymphocytic Leukemia (IWCLL) and National Cancer Institute (NCI) criteria for complete remission (CR), partial remission (PR), stable disease, or disease progression. Safety monitoring included World Health Organization (WHO) toxicity grading for all adverse events.
RESULTS: Seventy-eight (96.3%) of 81 recruited patients with previously treated B-cell chronic lymphocytic leukemia (CLL) received 10-mg tablets of fludarabine phosphate to a dose of 40 mg/m(2)/d for 5 days, repeated every 4 weeks, for a total of six to eight cycles. According to IWCLL criteria, the overall remission rate was 46.2% (CR, 20.5%; PR, 25.6%). The comparative figures using NCI criteria were 51.3% (CR, 17.9%; PR, 33.3%). Overall, 30 incidents of severe adverse events were reported for 22 patients. WHO grade 3 or grade 4 hematologic toxicities included granulocytopenia (53.8%), leukocytopenia (28.2%), thrombocytopenia (25.6%), and anemia (24.4%). Gastrointestinal adverse events were more common with the oral formulation than previously reported with IV fludarabine phosphate. However, these events were generally mild to moderate.
CONCLUSION: This study demonstrates that oral fludarabine phosphate has similar clinical efficacy to the IV formulation and a safety profile that is both predictable and essentially similar to that of the IV formulation.
PMID: 11709569
____________
Hematol J 2026;2(5):316-21
The bioavailability of oral fludarabine phosphate is unaffected by food.
Oscier D, Orchard JA, Culligan D, Cunningham D, Johnson S, Parker A, Klein M, Gieschen H.
Royal Bournemouth Hospital, Bournemouth, UK.
INTRODUCTION: A prospective, open and randomized, two-way crossover study was conducted to evaluate the pharmacokinetics and bioavailability of oral fludarabine phosphate when taken on a full versus an empty stomach. The effectiveness of therapy was also assessed after two cycles of treatment, four weeks apart.
MATERIALS AND METHODS: Patients with chronic lymphocytic leukemia or low-grade non-Hodgkin's lymphoma were randomly assigned to two groups, both of which received two cycles of treatment with 90 mg of oral fludarabine phosphate administered when either fed or fasted. Patients in Group 1 (n = 8) received oral treatment on a full stomach for the first cycle then on a fasted stomach for the second, while those in Group 2 (n = 10) received their treatment in the reverse sequence. Oral fludarabine phosphate was administered on the first day of the two study cycles and intravenous fludarabine phosphate was administered on days 3-6.
RESULTS AND CONCLUSION: Of 22 patients recruited, 18 (CLL n = 10; NHL n = 8) were eligible for efficacy and safety evaluation, and 16 for bioavailability and pharmacokinetic analyses. The response to oral 2-F-ara-AMP was rapid: by two treatment cycles, 12 out of 18 patients (66.7%) had achieved partial response. Of the six patients who did not respond, five patients (27.7%) had stable disease. There was no notable difference in the rate of response between patients with B-CLL and lg-NHL. There was a marginal increase in total systemic availability of fludarabine phosphate when administered orally on a fed stomach (2-F-ara-A AUC((0-24 h)) = 3.28 +/- 1.48 microg.h/ml) compared to a fasted stomach (2-F-ara-A AUC((0- 24 h)) = 3.05 +/- 1.56 microg.h/ml). Time to peak plasma concentration was slightly extended by the presence of food (2.2 +/- 1.0 versus 1.3 +/- 0.74 h) but the terminal half-life was unaffected. The minor differences in the pharmacokinetics of oral fludarabine phosphate when taken after food were not statistically significantly different and seem unlikely to be clinically relevant. The efficacy and safety data closely paralleled previous experience with the intravenous formulation.
PMID: 11920267
_____________

Fludarabine Contraindications
AIHA and ITP
Date: 12/11/02
by Chaya Venkat
There seems to be a significant amount of information available now that suggests Fludarabine is not the drug of choice, if there are concerns of autoimmune hemolytic anemia (AIHA) or idiotype thrombocytopenia (ITP). See two abstracts below from PubMed.
For many of us, once the watch and wait period is over and it is time to start therapy, low red blood cells, hemoglobin and hematocrit, as well as low platelets, are relatively common. These do not automatically suggest autoimmune diseases such as AIHA and ITP, but certainly these are complications that should be checked for. Certainly, reduced levels of these cell lines could be because the bone marrow is heavily compromised, and it is just not able to produce the right amounts of these cells. Autoimmune disease, on the other hand, is not because of bone marrow deficiency, but because the red blood cells or platelets are being destroyed before their time by an immune system gone berserk. It is relatively easy to test for this, and get an understanding on what is causing the anemia (low RBC) or thrombocytopenia (low platelets).
My question is this: how many local oncologists check for these potential complications prior to initiating Fludarabine based therapy? It seems to me that if the patient is susceptible to AIHA or ITP, Fludarabine is contra-indicated and some other therapy may be a better choice. I recognize that in heavily pre-treated patients there may be few choices left. But for frontline therapy, it hardly seems appropriate to initiate Fludarabine based therapy if there is indication of AIHA or ITP complications.
Perhaps a better choice in these cases is a monoclonal therapy such as Rituxan or Campath, with or without low dose corticosteroids. Especially in cases where the ITP is accompanied by enlarged spleen, Campath may be a good choice, since this monoclonal does a very good job on getting rid of tumor burden in the spleen. Perhaps frontline therapy with Rituxan and low dose Prednisone, followed by subcutaneous Campath to do the mop up job on the minimal residual disease?
If you are heading out to therapy for the first time, and you are concerned about potential complications of AIHA or ITP, it might be worth while to talk to your oncologist to rule out these potentially very dangerous complications, prior to making therapy choices. Especially if you are considering Fludarabine-based therapy, either as single agent or in combinations such as RFC or RF.
Hematol Cell Ther 1998 Jun;40(3):113-8
Severe autoimmune hemolytic anemia in eight patients treated with fludarabine.
Gonzalez H, Leblond V, Azar N, Sutton L, Gabarre J, Binet JL, Vernant JP, Dighiero G.
Service d'Hematologie, Hopital Pitie-Salpetriere, Paris, France.
We have used fludarabine to treat 36 patients with various lymphoid malignancies, including 29 with chronic lymphocytic leukemia (CLL). All these patients were heavily pretreated, and FAMP was prescribed on a compassionate basis. Eight patients (22%) developed severe autoimmune hemolytic anemia (AIHA) during or after treatment, and one died. Five patients had no previous history of hemolysis. These cases confirm the high incidence of AIHA after FAMP and suggest that the use of highly effective lymphocytotoxic agents such as fludarabine in heavily pretreated patients increases the risk of AIHA in CLL and other lymphoproliferative disorders.
PMID: 9698219
___________
Clin Lab Haematol 2026 Jun;22(3):175-8
Autoimmune thrombocytopenia: a complication of fludarabine therapy in lymphoproliferative disorders.
Leach M, Parsons RM, Reilly JT, Winfield DA.
Department of Haematology, Stobhill Hospital, Glasgow, UK.
We describe two cases of autoimmune thrombocytopenia precipitated by fludarabine therapy in patients with chronic lymphatic leukaemia. Both were treated with high dose steroids and initially responded with recovery of normal platelet counts. One patient developed recurrent autoimmune thrombocytopenia on two occasions following re- exposure to the drug when his disease had become refractory to all other treatments. A retrospective review of the case notes of 45 patients with lymphoproliferative disorders treated with fludarabine over the past 6 years indicated the development of autoimmune thrombocytopenia in 4.5% (two out of 45) and autoimmune haemolytic anaemia in 6.7% (three of the 45).
PMID: 10931169
____________
Fludarabine (Fludara)
Some Basic Background
Date: 4/29/02
by Chaya Venkat
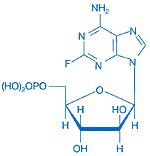 This is the third in my series of fact-sheets on frequently used chemotherapy drugs in the treatment of CLL. The usual disclaimer is in effect; this information is for the purpose of facilitating your discussions with your doctor, and not to be taken as medical advice.
This is the third in my series of fact-sheets on frequently used chemotherapy drugs in the treatment of CLL. The usual disclaimer is in effect; this information is for the purpose of facilitating your discussions with your doctor, and not to be taken as medical advice.
Fludarabine, along with pentostatin and cladribine, belong to the family of purine analogues. While the structures of all three of these compounds are similar, they do not all act in precisely the same way and have been found to show different clinical effects. Pentostatin (trade name Nipent) has been an effective drug in treating Hairy Cell Leukemia (HCL) but appears less effective against B-CLL, with overall response and complete response lower than treatment with fludarabine. Cladribine (trade name Leustat) requires frequent administration for optimal performance and since oral availability of the drug is only a third of IV administration, the intravenous route is the usual method of administration. In general cladribine remissions are impressive but short-lived.
Of the three purine analogues, fludarabine (trade name Fludara) is by far the best known and widely used for salvage therapy as well as front-line therapy for CLL. It is used both as a stand-alone drug and in combination with other drugs, such as the now-famous RFC (Rituxan, fludarabine and cyclophosphamide) combination. A new combination replacing fludarabine with pentostatin (Rituxan, pentostatin and cyclophosphamide or RPC) has been proposed for clinical trials by Dr. Mark Weiss at Memorial Sloan-Kettering Cancer Center.
This section is excerpted and condensed from Dr. Kanti Rai's book. (See reference below). If this section is too technical, feel free to skip it.
Fludarabine is rapidly transported into the cell through a series of dephosphorylation and rephosphorylation steps, and the active metabolite accumulates rapidly inside the cell within minutes after administration, soon reaching target levels required. The drug has multiple activities within the cell, not all of which are clearly understood. It inhibits DNA synthesis by competing with normal processes, and once incorporated into the DNA it is an effective chain terminator, producing DNA deletions, mutations and promoting general cell apoptosis. The activity of Fludarabine is not restricted to proliferating cells, and acts upon resting as well as proliferating lymphocytes. This is of particular interest in diseases such as CLL, which are not characterized by rapid cell proliferation. It is suggested that cell death occurs due to a preferential depletion of short-lived proteins.
Before you freak out reading the long list of side effects below, just remember, while Fludarabine is no pussy cat, it is one of the most powerful and effective chemotherapy drugs available today for treatment of CLL. The majority of us will likely have some use for this drug, either by itself or in combination with others. Killing cancer cells is no easy job, and until our researches develop effective and safe immunotherapeutic drugs and vaccines, Fludarabine will continue to play a very important role in treatment of CLL.
RxList on Fludarabine - - Excellent site, a great deal of very useful and in-depth information. If Fludarabine is in your immediate future, you should browse this site.
Cancer Bacup on Fludarabine - Good information, useful, brief and pragmatic.
Book — “Campath-1H Emerging frontline therapy in CLL” by Kanti Rai and Janet Stephenson.
Parthenon Publishing Group, New York.
This book is about 125 pages long, first two chapters provide a lot of background information about all the chemotherapy drugs used in CLL, including fludarabine. Chapters 3, 4 and 5 are devoted to monoclonal antibodies and Campath-1H. Quite technical, heavy reading but informative.
 Enter Keywords: |
———
Disclaimer: The content of this website is intended for information only and is NOT meant to be medical advice. Please be sure to consult and follow the advice of your doctors on all medical matters.
Copyright Notice:
Copyright © 2026-2007 CLL Topics, Inc. All Rights Reserved.
All materials contained on this site are protected by United States copyright law and may not be reproduced, distributed, transmitted, displayed, published or broadcast without the prior written permission of CLL Topics, Inc. You may not alter or remove any trademark, copyright or other notice from copies of the content.
However, you may download and print material from CLLTopics.org exclusively for your personal, noncommercial use.
———
