 |
||||||||||
Date: May 15, 2026
by Chaya Venkat
Related Article:
Review of Monoclonal Antibodies in Development

Two radioimmunotherapy drugs that have received a great deal of press in NHL but have seen almost no action in CLL, are Bexxar and Zevalin. Bexxar (tositumomab/iodine-131) and Zevalin (ibritumomab tiuxetan) are monoclonal antibodies targeting the CD20 marker, making them similar to Rituxan, but with the important difference that they both carry radioactive payloads. Both agents are approved in the United States for use in relapsed or refractory, indolent or transformed, B-cell lymphomas. Both drugs are well tolerated and have demonstrated the highest levels of single-agent activity observed in non-Hodgkin’s lymphomas.
Since NHL is also a B-cell cancer, a sort of kissing cousin of CLL, you may well ask why these wonder drugs have not been used in CLL. For a change, this was not merely due to CLL getting lost in the shuffle as researchers went after the larger prize of NHL therapies. There are indeed good reasons why radioimmunotherapy has been problematic in CLL (we will consider these later, but first, the punch line). An innovative clinical trial at the Fred Hutchinson Cancer Research Center (Seattle) has put Bexxar within reach of previously untreated CLL patients. This is an important trial and I would like to encourage all of you to familiarize yourselves with the concepts involved. Bexxar is a commercially available drug, within the reach of practicing oncologists and patients today. This is no pie-in-the-sky experimental drug that may take many years before it can be formally prescribed by oncologists. I have to confess, I am intrigued by this drug and this particular clinical trial. It offers a real alternative to those of you looking to get a solid remission right out of the gate with your frontline therapy.
Think of Bexxar as a sister drug of Rituxan and HuMax-CD20 (there are many articles on these two drugs, as you will find in the Rituxan Directory Page and the HuMax-CD20 Directory Page), since it too targets the CD20 marker carried by all mature B-cells. But Bexxar is a much more dangerous sibling, since it carries a far meaner punch — a radioactive payload. Bexxar is a murine antibody (entirely mouse protein), as opposed to Rituxan, which is chimeric (part mouse, part human), or HuMax-CD20, which is fully human. Bexxar is formed when the ‘naked’ anti-CD20 monoclonal antibody tositumomab is linked to a tiny amount of radioactive iodine, the Iodine-131 isotope to be specific. Never mind if you slept through the class in high school when they discussed the endlessly useful periodic table of elements. Suffice it to know that both the naked antibody and the radioactive iodine payload it carries are potent killers of cancer cells. The added punch of the radioactive dose, delivered with exquisite precision when CLL cells carrying CD20 markers are tagged, makes Bexxar an interesting “smart bomb”.
There is no free lunch, heck we all know that. So what are the advantages and disadvantages of radioimmunotherapy with drugs such as Bexxar? What are the risks and rewards? Here is a thumbnail sketch of how Bexxar stacks up.
By now we know quite a bit about how monoclonal antibodies like Rituxan and HuMax-CD20 carry out cell kill. (Here is a sampling of articles on the subject: A Smarter Monoclonal on Trial, Sons of Rituxan and Campath, Introduction to Monoclonal Antibodies, Review of Monoclonal Antibodies in Development). All of these mechanisms depend to some degree on getting assistance from the rest of the immune system, a tall order when we are talking about patients who have already been through numerous therapy regimens and whose immune systems are just not up to doing any heavy lifting. In the few instances where Rituxan does its own cell killing, without any help from the immune system, it is often a case of ganging up on the cancer cell. Literally thousands of Rituxan molecules festoon the target cell: direct cell kill by Rituxan does need this level of over-the-top Rituxan density on the target cancer cell.
Unfortunately for CLL patients, the typical number of CD20 markers per CLL cell is not very high, certainly not as high as it is in the case of non-Hodgkin’s lymphoma patients. That partially explains why Rituxan as a single agent is not anywhere near as effective in CLL patients as it is in NHL patients. We just cannot get enough Rituxan molecules to cover each CLL cell and this makes the job harder. Last but not least, the vast majority of CLL cells are not out in the peripheral blood circulation, where they can be easily attacked by Rituxan. When they hide out in swollen lymph nodes, the spleen, liver or bone marrow, CLL cells are a lot harder to kill. Kipps, et al., have suggested that in these locations the CLL cells are surrounded by “nurse-like cells”. The function of these nurse-like cells seems to be to encourage CLL cells to have more babies and be more resistant to cell kill. Oy vay! (I must have been Jewish in my last life, I find Yiddish so very expressive).
So, how does this situation change when the anti-CD20 monoclonal antibody carries its own lethal radioactive payload? For starters, in this case cell kill does not have to depend as much on getting help from the rest of the immune system. Bexxar can tag the cancer cell as well as Rituxan can, and then finish the job of killing it, all by itself. That is a huge advantage, when we are talking about late stage patients with compromised immune systems, or those with bulky disease and therefore heavy tumor load. “Naked” monoclonals like Rituxan and HuMax-CD20 may not do much for these guys but Bexxar can.
Second, it would seem that we do not need quite as many Bexxar molecules to tag each and every CLL cell. A lower number of Bexxar molecules per CLL cell is perhaps enough to do the job. Radioactivity does the job far better than hordes of Rituxan molecules literally smothering the CLL cell to death. Good news for us CLL folks. Even at the low or dim CD20 expression typical of our CLL cells, Bexxar may be able to do the job soup-to-nuts, tag and kill, all by itself.
Last but by no means least, Bexxar kills not only the particular CLL cell it has tagged. The power of the radioactive iodine it carries spreads a short distance beyond that one specific cell tagged. In other words, Bexxar kills the cells immediately surrounding the particular CLL cell to which it is attached. Several birds killed with one stone, as it were. Why is this important? It is suggested that this ability to kill clusters of cancer cells in one fell swoop makes Bexxar more effective in clearing out bulky lymph nodes choked with CLL cells living in tight proximity to each other. If in the process Bexxar also kills the nurse-like cells that are sleeping with the enemy, so much the better.
I hope as you read the last paragraph some warning flags went up in your head. Killing neighboring cells is good, when the neighbors are also bad guys that we want dead anyway. But what about unwanted collateral damage, friendly fire accidents we don’t want? What about the bone marrow and stem cells? Many of us have bone marrows that are significantly infiltrated with CLL cells. The CD20 markers on all these CLL cells in the marrow are tempting targets for Bexxar. With high CLL cell concentration in the marrow, the drug will enter and get concentrated in the bone marrow. True, the CLL cells in the marrow will get killed most efficiently. But along with that, there is real danger that Bexxar will also kill any precious stem cells and precursor cells that just happen to be in close proximity to the CLL cells. Killing off stem cells is not a good idea, not unless you plan to replace them soon after with a brand new immune system, as in a stem cell transplant. I will have more to say on the subject of using Bexxar in transplants, in a subsequent article.
This is the single biggest reason why Bexxar (along with its sister drug Zevalin) has not seen much use in CLL patients. We have far too much bone marrow involvement and attracting Bexxar to the bone marrow poses too much risk of collateral damage to precious stem cells. The design of the new clinical trial at the Hutch sidesteps this problem neatly, as you will see. But before we go there, let us examine the track record Bexxar has established in our sister disease, non-Hodgkin’s lymphoma.
PubMed has many citations of clinical trials using Bexxar (and Zevalin) to treat NHL patients. I thought I would limit myself to this one article cited below, since it is perhaps among the most credible. The patient cohort size was large, the results are published in a reputable journal (New England Journal of Medicine is about as blue-blooded pedigree as you can get) and I like to give my alma mater U of Michigan a plug whenever I can. Here is the abstract, write us if you want to read the full text of the article.
NEJM Article
N Engl J Med. 2026 Feb 3;352(5):441-9
131I-tositumomab (Bexxar) therapy as initial treatment for follicular lymphoma.
Kaminski MS, Tuck M, Estes J, Kolstad A, Ross CW, Zasadny K, Regan D, Kison P, Fisher S, Kroll S, Wahl RL.
Department of Internal Medicine, Division of Hematology and Oncology, University of Michigan Medical Center, Ann Arbor, MI 48109-0936
BACKGROUND: Advanced-stage follicular B-cell lymphoma is considered incurable. Anti-CD20 radioimmunotherapy is effective in patients who have had a relapse after chemotherapy or who have refractory follicular lymphoma, but it has not been tested in previously untreated patients.
METHODS: Seventy-six patients with stage III or IV follicular lymphoma received as initial therapy a single course of treatment with 131I-tositumomab therapy (registered as Tositumomab and Iodine I 131 Tositumomab [the Bexxar therapeutic regimen]). This consisted of a dosimetric dose of tositumomab and 131I-labeled tositumomab followed one week later by a therapeutic dose, delivering 75 cGy of radiation to the total body.
RESULTS: Ninety-five percent of the patients had any response, and 75 percent had a complete response. The use of polymerase chain reaction (PCR) to detect rearrangement of the BCL2 gene showed molecular responses in 80 percent of assessable patients who had a clinical complete response. After a median follow-up of 5.1 years, the actuarial 5-year progression-free survival for all patients was 59 percent, with a median progression-free survival of 6.1 years. The annualized rate of relapse progressively decreased over time: 25 percent, 13 percent, and 12 percent during the first, second, and third years, respectively, and 4.4 percent per year after three years. Of 57 patients who had a complete response, 40 remained in remission for 4.3 to 7.7 years. Hematological toxicity was moderate, with no patient requiring transfusions or hematopoietic growth factors. No cases of myelodysplastic syndrome have been observed.
CONCLUSIONS: A single one-week course of 131I-tositumomab therapy as initial treatment can induce prolonged clinical and molecular remissions in patients with advanced follicular lymphoma. Copyright 2026 Massachusetts Medical Society.
PMID: 15689582
____________
In a nutshell, 76 previously untreated but late stage (stage III or IV) follicular lymphoma patients participated in this trial. Bexxar was administered as frontline therapy. Now get this: there was just one infusion of Bexxar (not counting the mini-dose given earlier on to calculate customized dosage for each patient). Just one infusion. One trip to that comfy reclining chair in the doctor’s office. The overall response was an eye-popping 95%. Complete response (CR) was achieved by 75% of the participants, and 8 out of 10 of these folks were lucky enough to get a molecular remission (what we would call PCR negative remission).
We have seen similar statistics with combinations such as FCR, or FR. But those combinations take six cycles of drudgery, six months with innumerable trips to get infusions. Compare that with this one shot of Bexxar! With this level of potency, one would expect an equally potent level of hematological toxicity. Not so, to my pleasant surprise. It is hard to compare apples and oranges, but in my estimation the level of neutropenia, anemia and the like were significantly lower for Bexxar. We are used to higher levels of toxicity, deeper nadirs, and longer time for the toxicity to resolve, in chemo-immunotherapy combinations such as FCR and FR.
| Measure in 76 Patients | Absolute Neutrophil Count (ANC) | Hemoglobin (g/dL) |
Platelet Count |
| Mean nadir | 1,300 | 12.2 | 83,000 |
| Mean time to nadir (days) | 47 | 44 | 29 |
| Toxicity (%) | |||
| Grade 3 | 29 | 0 | 17 |
| Grade 4 | 5 | 0 | 0 |
| Duration of Toxicity (days) | 22 | 0 | 22 |
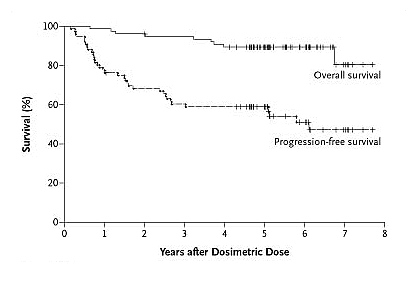
Now let us look at the robustness of the remissions. I don’t know about you, but I don’t particularly care what the remission statistics say immediately after the completion of therapy, what matters to me as a patient spouse is how long the remission is going to last, and will it help my guy live longer. Below is the graph that tells this part of the story. Remember, this is a group of advanced stage NHL patients, not your low risk just diagnosed crowd. Eyeballing the curves, you can see 90% or so of the patients were alive past the 6 year mark, and half of them were still in remission. I thought this was pretty impressive. How many of us would like to go for a therapy that gives a high probability of response, reasonably low toxicity, definitely low hassle and pretty good shot at a 6 year remission? The authors comment on the flattening of the disease-progression curve after three years. This is encouraging, suggesting that fewer patients are relapsing as time went on. Similar “plateaus” in survival curves are what makes stem cell transplants so tempting, the possibility that a segment of the patients may never relapse, dare we say the word, actually get cured? Most assuredly, longer follow-up is needed before we jump to any such conclusion. But one can always hope.
Survival After Bexxar Therapy
Kaminski, et al., NEJM, February 3, 2026
Since Bexxar uses radioactive iodine as the payload, it is important to consider thyroid issues. As you probably know, iodine is concentrated in the thyroid gland. Nine of the 76 patients had had an elevated level of thyroid hormone or had used thyroid medication before therapy began. Of the remaining 67 patients, 9 were found to have an elevated level of thyroid-stimulating hormone and 4 began thyroid medication after therapy. The authors estimate two-year cumulative incidence rates of elevated level of thyroid-stimulating hormone or needing thyroid supplementation was 8%. Something to consider, and something we do not normally think about with conventional CLL drugs.
Second Cancers after completion of therapy is something most studies ignore, the six-ton elephant in the living room no one wants to talk about. After more than 5 years of follow-up, roughly speaking, basal-cell carcinoma was diagnosed in one patient, breast cancer in two patients at 24 and 43 months, prostate cancer in one patient at 46 months, and ductal carcinoma in situ of the breast in one patient at 49 months. No cases of myelodysplastic syndrome or acute leukemia were observed after a median follow-up of 5.1 years.
This newly announced trial at the Hutchinson Cancer Center in Seattle (the “Hutch”) is quite innovative. It gets around the problem of heavy bone marrow involvement in CLL patients, and possible toxicity to stem cells if Bexxar is administered when the bone marrow is choke full of CLL cells by a simple trick. Patients are first put through a standard 6 cycle course of RF (Rituxan + fludarabine), which is generally sufficient to clean out the bone marrow in most CLL patients. Bexxar is given only after that pre-conditioning with RF, and making sure the bone marrow is sufficiently un-infiltrated, by means of a bone marrow biopsy. Innovative approach. However, I am afraid those kindly hematological toxicity numbers we saw above will be compromised, we can fully expect to get the standard dose of toxicity associated with RF therapy. I suppose the best comparison for this RF + Bexxar therapy would be a similar RF + Campath therapy, where the Bexxar and Campath are doing the job of minimum residual disease clearance.
Here is a pdf version of the patient consent form with more detail, including contact information.
Here is a quick list of the more important inclusion criteria. You can read the entire list of inclusion and exclusion criteria in the official listing of this trial in clinicaltrials.gov (NCT00476047) and get additional details by calling the contact phone numbers provided in the patient consent form above.
You can break down this protocol into two parts, the first part is RF therapy to get rid of the major bulk of the CLL, and even more important, make sure the bone marrow has been sufficiently cleaned out before Bexxar is administered. As we discussed above, heavy bone marrow infiltration by CLL cells means too much Bexxar will get attracted into the bone marrow, and if that happens, it can cause unwanted damage to the stem cells nearby.
The RF protocol used here is very similar to the one developed at Ohio State. You can look up a number of articles we have on the subject to get all the details (RF Therapy: Risks and Rewards; Fludarabine Monotherapy Is No Longer the Gold Standard, RF Therapy).
The dosages used are discussed in Drug Dosages in Popular Standard Therapies.
In brief, each cycle consists of fludarabine infusion (25 mg/m2) on days 1, 2, 3, 4 and 5, plus a single infusion of Rituxan on either day 4 or 5 of the same week, depending upon convenience. Then you get a break for four weeks, and the whole process repeats for the next cycle. You get a total of 6 such cycles. Doing a little math, that adds up to 25 weeks, from beginning of the first cycle to end of the last cycle in this RF part of the protocol.
The cool stuff starts after the RF is over. Bexxar will not be given for at least 90 days after the last RF cycle is complete. Even then, it will be given only if peripheral blood counts have recovered. If by day 120 after completion of the RF part of the trial your counts are still sucking wind big time, "Hasta la vista, Baby!", you are off of the study. This is something to bear in mind as you contemplate this study. Frankly, I am relieved this safety valve is built into the study. Bexxar is probably not a good thing to give people whose blood counts have tanked after RF therapy and are taking too long to recover, suggesting they do not have a bone marrow that is ready for more therapy. Here are the major requirements before you get the Bexxar part of the trial:
Garden variety drug dosages are often customized by simple methods reflecting the size of the patient’s body. For example, the standard infusion dose of Rituxan is 375 mg/m2. In the case of Bexxar, getting the exact dose customized to each patient is a little bit more fancy than just measuring the weight and height of the patient and calculating the BSA (“body surface area”). The first infusion of Bexxar on day 0 is called the dosimetric infusion, just a tiny bit of the drug infused to see how your body handles it, how it is absorbed and eliminated. Your body will be scanned three times in the week after the dosimetric infusion, to get a good picture of how the drug’s radioactivity interacts with you. Based on the data from the scans, two weeks from the day you get the sneak preview dosimetric infusion, the specific dose of Bexxar customized to be right for your body (amounting to a total of 75 cGy radiation) will be given. Not counting the dosimetric infusion, the therapeutic dose of Bexxar is given in just one infusion, one day, that’s it. You are done. Welcome relief from the 6 month long drawn out affairs we have gotten to expect with RF, FCR etc.
Administering Bexxar is a bit more complicated that just hooking you up to the infusion pole, and monitoring your blood pressure once every little while, if you are lucky and have detail-oriented nursing staff doing the infusion. Radiation is not something to kid about and Bexxar is radioimmunotherapy, no kidding. You won’t glow in the dark or anything cool like that, but precautions are necessary to make sure the correct dose is given to each patient. I heard rumors that you are advised to use a different bathroom than your spouse, since much of the Bexxar is finally eliminated in urine. And no, your pee will not turn blue, no matter what the rumor mill says. Sorry, you guys. (Yes, it would have been ever so cool if it did turn blue, and ![]() in the dark.) If any of you are considering doing this protocol off-study on your own, remember that Bexxar can only be administered by hospitals and doctors that have been trained and approved to do so.
in the dark.) If any of you are considering doing this protocol off-study on your own, remember that Bexxar can only be administered by hospitals and doctors that have been trained and approved to do so.
Radiation therapy to treat “solid” cancers has a long track record. When all the cancer cells are nicely restricted to one location, say in early stage breast cancer before it has metastasized, radiation therapy makes a lot of sense. Radiation focused carefully at the right spot kills only the cancerous tissue, perhaps a little bit of the surrounding cells, but not much more. Major organs are spared radiation toxicity. The majority of breast cancer patients get a combination of chemotherapy, radiation and surgery.
This scenario just does not work in blood cancers such as CLL. By definition, blood cancers are every where in the body, every where that blood flows, right from the start. How can we give radiation that is restricted to just the cancer cells and not poison crucial organs such as the heart, when the darned cancer cells are everywhere? Until recently, radiation has played a very minor role for CLL, mostly limited to small doses to swollen nodes when they become too large for comfort. Drugs such as Bexxar have changed that. Now we can use the talent of monoclonal antibodies, their very single minded and specific ability to bind to the marker expressed by cancer cells. In this case, the ability to seek out and tag only cells that contain the CD20 marker, expressed only by mature B-cells. The radioactive payload of Bexxar goes along for the ride. In this fashion we are finally able to focus radiation exactly where it is needed, very fine tuned focusing indeed, at the molecular level.
So, what do the researchers expect to learn from this study? We already have a fair understanding of how chemo-naïve CLL patients respond to RF therapy – Byrd, et al., have done a terrific job of documenting this combination. The new wrinkle in this study is the addition of Bexxar. The primary objective is to measure the percentage of patients who have not had disease progression past the 2 year mark. I fully expect we will see longer remissions, higher percentage of patients who remain without disease progression beyond the 2 year mark, when we compare RF with or without Bexxar chaser. The track record of very long remissions established in NHL is certainly impressive, and there is reason to hope it will do as well in our patient population as well.
However, the million dollar question is which patients benefit the most from this combination? As we have already seen in the Ohio State work with RF therapy, not all CLL patients respond the same way. Patients in good prognostic buckets get far more CRs (“complete response”) than patients in the high risk buckets. (What Type of CLL Do You Have?). Even when high risk patients get CRs, the remissions do not last as long, and the disease comes roaring back sooner than we would like. We discussed this important issue in a past article, RF Therapy: Risks and Rewards.
I sincerely hope the researchers plan to obtain prognostic information on the patients they recruit (IgVH gene mutation status and FISH cytogenetics at the very least), and break out the response statistics in terms of prognostic risk buckets. I know, this is easier said than done. It is hard enough to recruit enough patients to do the overall clinical trial, large enough sample size to derive statistically robust answers. Once we start slicing and dicing the patient cohort into smaller subsections based on prognostics, very soon we get onesies and twosies, and that plays havoc with the statistical value of the results – the conclusions are little more than anecdotal.
Nevertheless, this kind of information is essential as we go forward in developing better options for our patients. In a previous study using Zevalin (sister drug to Bexxar, another anti-CD20 monoclonal with radioactive payload) the authors hypothesize that patients with defective 17p (p53) may not have responded as well as those without this defect. Will we see the same thing with Bexxar? Will the folks with high risk FISH cytogenetic abnormalities, such as the infamous 11q (ATM) and 17p (p53) deletions, fare worse than patients without these crucial defects?
Frankly, if Bexxar consolidation after RF therapy fails to get robust remissions for these high risk Bucket C folks, I would be disappointed. We have several therapy options for the good prognosis cases, they respond well and get long remissions from just about any of the chemo-immunotherapy regimens such as RF, FCR, PCR etc. It is the Bucket C kids that I worry about, perhaps because my own husband belongs to that high risk group. The hope is that addition of Bexxar as a way of cleaning out minimum residual disease after RF will give good long remissions to the high risk patients as well. Only time will tell if is the case. At the very least, this innovative clinical trial gives us a chance of finding out if Bexxar has a role to play in treating high risk CLL.
 Enter Keywords: |
———
Disclaimer: The content of this website is intended for information only and is NOT meant to be medical advice. Please be sure to consult and follow the advice of your doctors on all medical matters.
Copyright Notice:
Copyright © 2026-2007 CLL Topics, Inc. All Rights Reserved.
All materials contained on this site are protected by United States copyright law and may not be reproduced, distributed, transmitted, displayed, published or broadcast without the prior written permission of CLL Topics, Inc. You may not alter or remove any trademark, copyright or other notice from copies of the content.
However, you may download and print material from CLLTopics.org exclusively for your personal, noncommercial use.
———
