 |
||||||||||
Date: July 28, 2026
by Chaya Venkat
Related Articles:
CLL Topics Sponsored Projects
GVHD: Graft versus Host Disease
GVHD — A Pebble in the Transplant Shoe
Sons of Rituxan and Campath
Immunomodulators & Rituxan
Harvey's Journal

If you are frequent visitors to the CLL Topics website or Harvey's Journal, you will understand I have not had a lot of time to publish new articles in the last several months. But I am happy to report I have not closed my eyes or mind to new opportunities that happen to come by, new technology that might be of tremendous value to our patient community. Today I would like to tell you about a brand new clinical trial that has already launched at the University of Minnesota, one that might give patients a shot at getting the much desired graft-versus-leukemia (GVL), while steering clear of the dreaded Graft-versus-host disease (GVHD). The good news is that CLL patients are eligible for this trial. I am so impressed by the concept of this trial and its potential that we have decided to fund part of the cost of this trial with your generous and precious dollar donations. Right there is a strong vote of confidence, our good housekeeping seal of approval as it were.
With the advent of “mini-allo” (same as “non myeloablative”, “reduced intensity conditioning”) transplant procedures, the risk of death due to heavy duty preconditioning has been greatly reduced. Likewise, transplant centers are getting much better at identifying and treating potential infections while the patient’s brand new immune system comes up the learning curve. That is not to say that stem cell transplants have become a slam dunk procedure without significant risk of death and misery. One of the major risks associated with allogeneic transplants is the dreaded GVHD (Graft-Versus-Host Disease).
In a nutshell, graft-versus-host-disease happens when the new immune system coming in (the “graft”) cannot tell the difference between friend and foe and starts attacking perfectly good cells and tissue of your body (the “host”). Unfortunately, damping down this over zealous response of the new immune system by means of immune suppressing drugs also puts the brakes on the ability of the graft to kill the remaining traces of cancer cells in your body. Without GVL (Graft-Versus-Leukemia) there would be no reason to expect a CURE of the underlying CLL. In the stem cell transplant context, GVHD and GVL are two sides of the same coin and researchers have been struggling to minimize the GVHD while boosting the effects of GVL. This high wire act is the Holy Grail of all transplanters everywhere.
Now it looks like we might be making headway on this important front, based on better understanding of the mechanisms involved in GVHD and GVL. It seems the major players involved in causing most of the damage of GVHD are T-cells. T-cells are among the premier “smart” troops of our immune system, serial killers that can methodically attack cell after cell with no mercy. Much of the research aimed at controlling GVHD has focused on controlling the activity of newly engrafted T-cells.
So, if T-cells are the big brutes on a rampage of GVHD, what cell type is most involved in activating precious GVL? New research findings point to NK cells as the most potent cell line capable of producing robust GVL. As their name implies, “Natural Killer Cells” are just that – cells whose main function in life is to kill pathogens and cancer cells. And here is the important bit, NK cells do not seem to be as interested in doing GVHD mayhem. I can see the light bulbs go off in the heads of many of our readers – can we get a transplant of donor NK cells, just enough to kill all traces of CLL in our bodies via potent GVL, but be sure not to use donor T-cells to trigger nasty GVHD side effects? Once the job is done, is it possible for our bodies to bid a polite good bye to the foreigner NK cells as well, send away the “mercenaries” once they have accomplished their mission?
That my friends, is exactly what this clinical trial attempts to do – keep the cancer curing aspects of stem cell transplants, while minimizing the risks of GVHD. And at the end of it all you will still keep the immune system you were born with, but potentially cured of the cancer that was buggering it up. How sweet is that? Another way of understanding this concept is to think of it as a combination of the best features of autologous transplants (where no donor is involved, you keep your own immune system and there is much less risk of GVHD) with the potent, curative potential of an allogeneic transplant (the donor NK cells are more likely to mount GVL attack on your cancer cells, a case of NK cells uncorrupted by prior cozy relationships with CLL cells). With this concept you may get the best of both worlds, as it were.
Our immune systems are incredibly complex and inter-related beasts. Nothing about the immune system is simple, or adequately described in short sound bites. Most of us are not hematologists or immunologists. Nevertheless, we need to understand some of the logic that went into this clinical trial, what makes it such an intriguing one, before we commit our hard earned cash or our even more precious bodies to it. I will be the first one to admit my review of this technology is superficial at best. The idea is to lay out the technology in broad terms, so that you can get a sense of the lay of the land. If you are interested in learning more about it, please write to me and I will see what I can do to get you detailed information, or at least put you in touch with folks that can give you more detailed information.
Below is our cheat-sheet version of what is involved in the NK-cell study.
Reducing the tumor load as much as possible ahead of NK-cell infusion is necessary, so that the new donor NK cells coming in are not out-numbered by huge hordes of cancer cells. Most of us are only too familiar with fludarabine and cyclophosphamide. While they are a powerful combination in reducing tumor load, they are also infamous for drastic reduction of your immune defenses because these drugs are also excellent killers of T-cells. Turns out, that is exactly what is needed here. Drastic reduction in the number of defenders (T-cells) in your body is essential to the survival of the “foreign” NK cells in your body long enough to do some good. If your own immune system is too strong, if there are too many host T-cells around, they will kill the precious donor NK cells in record quick time. Last but not least, when your body senses that it has very few lymphocytes of any kind (T-cells, B-cells, NK-cells) left behind after the chemotherapy, it does its best to cherish and make any new lymphocytes still around feel as comfortable and right at home as possible. After lymphocyte reduction with chemotherapy your body lays out the welcome mat as it were, with a host of nurturing cytokines that encourage new NK cells coming in to multiply and stick around for a few weeks, hopefully long enough to do a good job of GVL.
This is a significant advantage of the haplo NK-cell therapy approach. I know of too many CLL patients without access to fully matched adult donors, whether they be sibling donors or matched unrelated donors. For people of non-cookie cutter ethnicity (like Harvey), it is next to impossible to find a well matched donor for a full stem cell transplant. On the other hand, most of us have kids and each and every one of our kids is a certified haplo donor. Here is a chance for the kid to pay back for all those bills to the orthodontist, not to mention college.
Many CLL patients have used single agent Rituxan as a therapy option in early stages of their disease. Rituxan is a monoclonal antibody that attaches itself to the CD20 markers exhibited by mature B-cells, and only mature B-cells. One of the ways by which Rituxan kills B-cells (CLL cells) is by a mechanism called ADCC (antibody dependent cellular cytotoxicity). In simple terms, the mere fact that the CLL cell is festooned with thousands of Rituxan molecules anchored to the cell via CD20 markers attracts the attention of killer cells, especially NK cells. Now you see the need for the last two infusions of Rituxan. Tagging the remaining CLL cells with Rituxan paints a bulls-eye on them, making them attractive targets for killing by the haplo NK cells. You can learn more about ADCC and other mechanisms by which drugs such as Rituxan and Humax-CD20 kill CLL cells by reading an earlier article on our website: How Monoclonals Work.
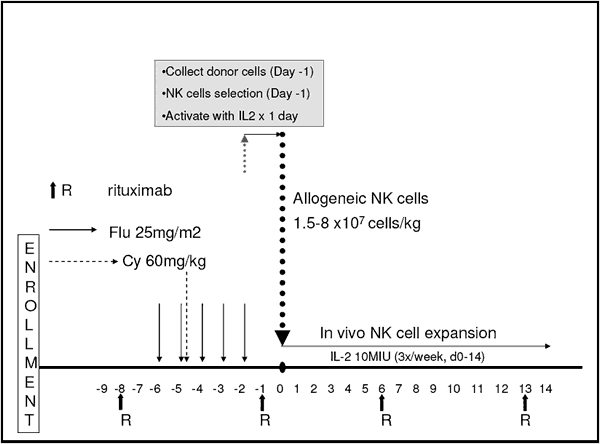
This trial is seeking to establish exactly how deep the clearance of cancer cells will be: will it be enough to give long lasting remissions? Dare we hope it will be powerful enough to bring about actual cures for even a subset of patients? Only time will tell how well this approach plays in the real world. That is what makes this clinical trial so very important, it has the potential for harnessing very powerful and possibly curative effects of GVL, without mandating that the patient’s original immune system is completely destroyed and replaced with a full immune system from a matched donor. By the way, as I am sure you have figured out by now, nothing prevents you from going the route of a full fledged mini-allo transplant after undergoing the NK-cell approach first. In fact, the NK cell therapy may be one way for patients with truly refractory CLL to get a clean remission ahead of a mini-allo transplant, patients who otherwise have no way of getting a clean remission.
As we mentioned above, this trial is open to CLL patients and NHL patients. CLL patients are eligible to participate if they have had fludarabine containing therapy at least twice, and are now in need of therapy once more. You must have hemoglobin levels higher than 9.0, platelets higher than 80K, neutrophil count better than 1.0K. Liver, kidney, heart and lung function need not be at Olympic athlete level, but not deteriorated to the point of being a basket case. If you are pregnant or breast feeding, forget about it. Allergic reaction to Rituxan is an automatic disqualifier as well, as is HIV disease. Of course, you must have a suitable haplo matched NK cell donor. For most of us that boils down to having a kid of our own (not step children or adopted children).
The full title of this trial is “Allogeneic Natural Killer Cells With Rituximab In Patients With CD20 Positive Relapsed Non-Hodgkin Lymphoma or Chronic Lymphocytic Leukemia”, offered only at the University of Minnesota (Minneapolis). The trial number is MT2007-12. Principal investigator is a charming young researcher named Dr. Veronika Bachanova. PC and I had the pleasure of getting to know Dr. Bachanova well while he was undergoing his cord blood transplant at Minneapolis. To say that we were impressed by her and this clinical trial is an understatement.
This trial opened just a few months ago, in January 2026, and only two patients have been put through the full process. I had the honor of meeting the first volunteer who participated in this trial, as well as his lovely wife. Don and Rose, folks like you are the real heroes of clinical research and we wish Don continued good health in the months and years to come. Some day, because of the courage of patients like you, we will find better therapy options and long lasting cures for all CLL and NHL patients.
This clinical trial is listed on www.clinicaltrials.gov, as should all legitimate clinical trials in this country. Here is the link to the citation: NCT00625729. Bear in mind this is an early phase I/II clinical trial and plans to recruit only 12 volunteers. Contact information is given below:
|
Natural Killer Cells With Rituximab In Patients With CD20 Positive Relapsed Non-Hodgkin Lymphoma or Chronic Lymphocytic Leukemia ClinicalTrials.gov Identifier: NCT00625729 Clinical Trials Office – Masonic Cancer Center at University of Minnesota Principal Investigator: Dr. Veronika Bachanova |
If after reading through this article you think you might be interested in participating in this clinical trial, write to me if you need additional information. Or, you may wish to contact the University of Minnesota or Dr. Bachanova directly at the phone numbers above. Be sure to tell 'em CLL Topics sent you - after all, you are part "owner" of this clinical trial!
 Enter Keywords: |
———
Disclaimer: The content of this website is intended for information only and is NOT meant to be medical advice. Please be sure to consult and follow the advice of your doctors on all medical matters.
Copyright Notice:
Copyright © 2026-2007 CLL Topics, Inc. All Rights Reserved.
All materials contained on this site are protected by United States copyright law and may not be reproduced, distributed, transmitted, displayed, published or broadcast without the prior written permission of CLL Topics, Inc. You may not alter or remove any trademark, copyright or other notice from copies of the content.
However, you may download and print material from CLLTopics.org exclusively for your personal, noncommercial use.
———
