 |
||||||||||
Date: November 9, 2026
by Chaya Venkat
Related Article:

Clinical trial statistics have become very important in recent years and many researchers use high response rates as their claim to fame. There may be some merit to this: it seems intuitively obvious that high response rates will also mean longer and healthier remissions. Intuitive, but not always accurate. Going after the CLL hammer and tongs, hitting it with multiple drug combinations may indeed increase the response rates, but there is also the risk that the increased response rates may come at the expense of higher rates of infections, hospitalizations, secondary cancers and indeed no improvement in the overall survival. It may be a case of winning the battle but losing the war. Getting that coveted "PCR negative" response would be a bitter victory if the remission did not last very long and you were back in the game a lot sooner than you hoped.
In the first place, one needs to examine these response rates carefully. You know the saying there are lies, damned lies and then there are statistics. Without a full-blown evaluation of the statistics and a detailed comparison of the trial designs, comparisons of response rates between different clinical trials is not only meaningless, it can lead to wrong therapy choices. Is RF (Rituxan + fludarabine) better than FRC? Does the addition of "C" (cyclophosphamide) increase the overall response rates of the FRC combo therapy over and beyond the results obtained by RF? Who knows. We and our doctors will be able to make better judgments on that front when M. D. Anderson publishes their long-running FRC clinical trial data. I hear the FRC trial inducted many more chemo-naive and earlier stage patients than the Ohio State RF trial, and the slightly higher response rates of the former may reflect the fact they had an easier crowd of patients to deal with. My favorite mantra: the devil is in the details. We shall see if this was indeed the case when the long awaited Anderson paper comes out. I understand it is going to happen soon, and I for one am looking forward to reading all about it.
But there is something else to think about here. Response statistics do not really address the real question. To be blunt about it, as patients we do not really care what the good doctors call our particular response. That alphabet soup of CR, NPR, PR, PD, PCR etc. means squat, the real question that interest us is this: are we going to live longer, with fewer complications and a better quality of life, by choosing Therapy A versus Therapy B? I will be the first to admit, this is not an easy question to answer in CLL. Researchers can come up with tabulations of response rates after finishing the clinical trial and perhaps monitoring the patients for a few more months. But overall survival is a much harder and certainly more expensive question to answer. Since CLL is an indolent disease, patients live for several years with or without (and sometimes in spite of) therapy. Survival statistics can only be obtained by very long term follow-up, which is both expensive and not very exciting. Response statistics are easier to get, and yield more splashy sound bites.
Here is one clear-cut example of why looking only at response rates can give very misleading information if what interests you is how much time you have to live and the quality of that life. Chlorambucil has been the standard of therapy for CLL patients in past decades and it is still used as frontline therapy in many countries. When fludarabine came along, this new drug (a purine analog) became the new darling, rapidly gaining acceptance as the new "gold standard" as frontline CLL therapy. And indeed, the response rates were impressive with this new drug. The abstract below is one of the several pivotal articles that compared these two drugs. A large cohort of chemo-naive patients (509 of them) were randomly assigned to get fludarabine or chlorambucil as front-line therapy. (There was also an arm of the trial that had patients getting a combination of fludarabine + chlorambucil, but this arm was shut down because there were too many infections with this combination). Sure enough, many more patients got a good response with fludarabine than with chlorambucil.
| Response | CR | PR | Total |
| Fludarabine | 20% | 43% | 63% |
| Chlorambucil | 4% | 33% | 37% |
| Statistically Significant? | Yes | Yes | Yes |
Well, that seems very clear, fludarabine wins hands down, right? Who can quarrel with 63% overall response rate, compared with 37%? And did you look at that difference in the "CR" (complete response) rates? Wow. Fludarabine beats chlorambucil, big time. The choice is clear that patients should go for fludarabine over chlorambucil, if these were their two choices for front-line therapy, right?
But in reality, the choice is as clear as mud. If you bothered to read on, you might come up with an entirely different conclusion. The median overall survival of patients in the fludarabine arm was 66 months, compared to 56 months for the chlorambucil arm. The difference between the fludarabine arm and chlorambucil arm were statistically insignificant, in other words, within the margin of error. Therefore, if long life was your objective, the results were a toss-up as there was no way of picking the winner between these two drugs. But … here is another piece of information that might interest you as the patient, the player with the most skin in the game: severe infections and neutropenia were more frequent, with statistical significance, among the patients who got fludarabine, compared to those who got chlorambucil. As far as patients are concerned, response statistics are not all that important – not when there is no improvement in overall survival – and especially if those sexy response statistics are bought at the expense of more infections and a poorer quality of life. Judging on that more real-life basis, the bottom line here is that fludarabine is not such a slam dunk after all!
Let us look at some other issues that are important to patients. Chlorambucil is a cheap drug: the doctor gives you a prescription for tablets that you take orally at home. Fludarabine is most often given as an intravenous infusion, administered at the hospital or doctor's office. It is a lot more expensive, especially if you add in the cost of high-priced nursing staff, etc. If you happen to be an older individual, for example, living in a rural area and find it hard to get to the hospital daily for five days out of every 28 days, and repeat that month after month as in this clinical trial (12 months was the longest), or you have poor quality health insurance coverage, just the cost as well as the ease of taking chlorambucil at home may make it start looking better. I need to add one caveat to this aspect of the comparison between the two drugs: fludarabine tablets have recently made their debut in Europe, which takes away the need for intravenous infusions and daily trips to the hospital. But the FDA has not yet approved their use in the U.S.A., so for now we do not have that option.
| Therapy | Survival | Infections/ Neutropenia |
Cost | Administration |
| Fludarabine | 66 months | More Often | Expensive | Intravenous |
| Chlorambucil | 56 months | Less Often | Cheap | Tablets (oral) |
| Statistically Significant? | No |
Editor's Note: Since the date of this article, fludarabine has come off patent in the US and elsewhere and may one day become available in generic form, like chlorambucil, and therefore cheaper. Schering A. G., the former holder of the fludarabine patent, markets the oral version of the drug overseas. Schering's U. S. subsidiary has not been successful in getting oral fludarabine made available in the U. S., ostensibly as a result of the FDA's reluctance to accept data from overseas clinical trials. A generic form of the drug is still possible, from some other manufacturer. However, some drug manufacturer still has to make an application to the FDA to have an oral form of the drug approved for sale in the U. S., with clinical data to back up claims of safety and efficacy – all of which costs money. Little wonder that in mid-2007 fludarabine is still an important drug in CLL treatment but can still only be administered in the U. S. as an intravenous infusion in an oncologist's office, with all the inconvenience and costs that that mode of administration involves to patients.
Overall Survival According to Treatment Group
Rai, et al., New England Journal of Medicine
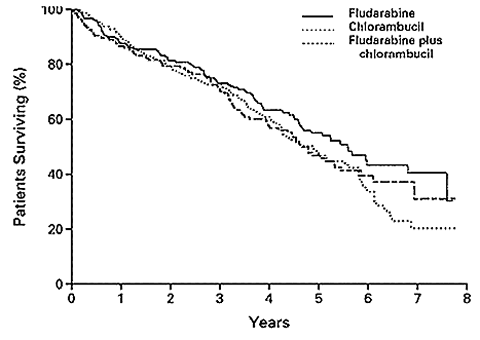
The moral of the story: if you are getting ready for your first therapy, and your two therapy choices are fludarabine or chlorambucil as single agents, fludarabine is not a slam dunk choice. There are issues here that you need to discuss with your doctor. In addition to reading the abstract below, if you would like to read the full-length article, please write to us and we will help you locate it. It could make your discussion with your doctor more focused and productive. You might also want to read the second abstract below. That one discusses the risk of secondary cancers and possible transformation of the original CLL to more aggressive variants, caused by fludarabine / chlorambucil therapy. As usual, write to us if you want to find the full text of this article.
N Engl J Med. 2026 Dec 14;343(24):1750-7
Fludarabine compared with chlorambucil as primary therapy for chronic lymphocytic leukemia.
Rai KR, Peterson BL, Appelbaum FR, Kolitz J, Elias L, Shepherd L, Hines J, Threatte GA, Larson RA, Cheson BD, Schiffer CA.
Cancer and Leukemia Group B, Chicago, IL.
BACKGROUND: Fludarabine is an effective treatment for chronic lymphocytic leukemia that does not respond to initial treatment with chlorambucil. We compared the efficacy of fludarabine with that of chlorambucil in the primary treatment of chronic lymphocytic leukemia.
METHODS: Between 1990 and 1994, we randomly assigned 509 previously untreated patients with chronic lymphocytic leukemia to one of the following treatments: fludarabine (25 mg per square meter of body-surface area, administered intravenously daily for 5 days every 28 days), chlorambucil (40 mg per square meter, given orally every 28 days), or fludarabine (20 mg per square meter per day for 5 days every 28 days) plus chlorambucil (20 mg per square meter every 28 days). Patients with an additional response at each monthly evaluation continued to receive the assigned treatment for a maximum of 12 cycles.
RESULTS: Assignment of patients to the fludarabine-plus-chlorambucil group was stopped when a planned interim analysis revealed excessive toxicity and a response rate that was not better than the rate with fludarabine alone. Among the other two groups, the response rate was significantly higher for fludarabine alone than for chlorambucil alone. Among 170 patients treated with fludarabine, 20 percent had a complete remission, and 43 percent had a partial remission. The corresponding values for 181 patients treated with chlorambucil were 4 percent and 33 percent (P< 0.001 for both comparisons). The median duration of remission and the median progression-free survival in the fludarabine group were 25 months and 20 months, respectively, whereas both values were 14 months in the chlorambucil group (P<0.001 for both comparisons). The median overall survival among patients treated with fludarabine was 66 months, which was not significantly different from the overall survival in the other two groups (56 months with chlorambucil and 55 months with combined treatment). Severe infections and neutropenia were more frequent with fludarabine than with chlorambucil (P=0.08), although, overall, toxic effects were tolerable with the two single-drug regimens.
CONCLUSIONS: When used as the initial treatment for chronic lymphocytic leukemia, fludarabine yields higher response rates and a longer duration of remission and progression-free survival than chlorambucil.
_____________
J Clin Oncol. 2026 Sep 15;20(18):3878-84.
Therapy-related myeloid leukemias are observed in patients with chronic lymphocytic leukemia after treatment with fludarabine and chlorambucil: results of an intergroup study, cancer and leukemia group B 9011.
Morrison VA, Rai KR, Peterson BL, Kolitz JE, Elias L, Appelbaum FR, Hines JD, Shepherd L, Larson RA, Schiffer CA.
Section of Hematology/Oncology, Veterans Affairs Medical Center, Minneapolis, MN 55417
PURPOSE: Patients with chronic lymphocytic leukemia (CLL) may have disease transformation to non-Hodgkin's lymphoma or prolymphocytic leukemia; however, development of therapy-related acute myeloid leukemia (t-AML) is unusual. A series of patients enrolled onto an intergroup CLL trial were examined for this complication.
PATIENTS AND METHODS: A total of 544 previously untreated B-cell CLL patients were enrolled onto a randomized intergroup study comparing treatment with chlorambucil, fludarabine, or fludarabine plus chlorambucil. Case report forms from 521 patients were reviewed for t-AML.
RESULTS: With a median follow-up of 4.2 years, six patients (1.2%) to date have developed therapy-related myelodysplastic syndrome (t-MDS; n = 3), t-AML (n = 2), or t-MDS evolving to t-AML (n = 1), from 27 to 53 months (median, 34 months) after study entry. This included five (3.5%) of 142 patients treated with fludarabine plus chlorambucil and one (0.5%) of 188 receiving fludarabine; no chlorambucil-treated patients developed t-MDS or t-AML (P =.007). At study entry, the median age among these six patients was 56 years (range, 44 to 72 years); three were male; the CLL Rai stage was I/II (n = 4) or III/IV (n = 2). Response to CLL therapy was complete (n = 4) or partial remission (n = 1) and stable disease (n = 1). Marrow cytogenetics, obtained in three of six cases at diagnosis of t-MDS or t-AML, were complex, with abnormalities in either or both chromosomes 5 and 7. Other abnormalities involved chromosomes X, 1, 8, 12, 17, and 19. Median survival after diagnosis of t-MDS/AML was 3.5 months (range, 0.5 to 10.1 months).
CONCLUSION: Our findings raise the possibility that alkylator-purine analog combination therapy may increase the risk of therapy-related myeloid malignancies, which is of particular relevance with regard to ongoing trials using these combination therapies.
PMID: 12228208
_____________
In a recent article (Fludarabine Monotherapy No Longer the Gold Standard) we reviewed fludarabine (F) versus a combination of Rituxan + fludarabine (RF) in the frontline treatment of CLL. What makes the Byrd, et al., paper that we reviewed so powerful is that they did not stop at merely discussing response stats – they actually went on to discuss survival rates. Not only did more patients get a solid response to RF combination therapy compared to F, there was a significant and robust improvement in the overall survival, even with just a few years of follow-up. My bet would be that this difference is real and will get only more substantial as time goes on. For a change, the research report makes our therapy choices clear: if you are trying to decide between RF versus F, the smart money would be on RF, for the average CLL patient.
I hope at least a few readers caught that little caveat, "the average CLL patient". By now we hope we have trained you to be suspicious, to realize that you need to be quick to catch these little CYA-type ifs and buts. We all know there is no such thing as an "average CLL patient". We are all as unique as they come and the big news these days is that one size does not fit all CLL patients. One of the breakthroughs has been the use of FISH analysis to determine the nature of the cytogenetic abnormality that is driving the CLL (What Type of CLL Do You Have?). The conventional wisdom of Watch & Wait is making way for prognostics-based therapy choices (Need to Know – aka Mayo Best Practices). I just wish these changes were taking place more quickly. The agonizingly slow pace of information trickle-down from research experts to the local level does not match our needs as patients. As individuals trying to live with this disease we are forced to march to a faster drum beat.
Here then is the million dollar question: how does RF therapy work in CLL patients with different risk factors, especially as defined by FISH cytogenetics? I was very pleased to find that as continuation of their excellent paper comparing RF versus F, Byrd, et al., have an abstract in the 2026 ASH (American Society of Hematology) Annual Meeting, where they expanded their study results. This important abstract is given below. Once again, I would like to urge you not to look too long at the response statistics and instead pay more attention to the length of the remissions. "PFS" stands for progression free survival. Not enough time has passed for us to get a handle on overall survival and we have to be satisfied with the length of the remission for now. As patients with families to raise and mortgages to pay, we care less about the academic name tag associated with the response, whether it is a CR or NPR or whatever. What really counts is how long the remission lasts, how long you can go on with your normal daily life without needing additional therapy for the CLL. As you can see from the table below, FISH cytogenetics has a great deal to say on this subject. People with the Bucket C cytogenetics of deletion of 17p or deletion of 11q (ATM) have shorter remissions after RF therapy, compared to patients with better FISH cytogenetics.
ASH 2026, Abstract # 476
Select High Risk Genetic Features Predict Earlier Progression Following Chemoimmunotherapy with Fludarabine and Rituximab in Chronic Lymphocytic Leukemia (CLL): Preliminary Justification for Risk-Adapted Therapy.
Session Type: Oral Session
John C. Byrd, John G. Gribben, Bercedis Peterson, Michael R. Grever, Gerard Lozanski, David M. Lucas, Richard A. Larson, Michael A. Caligiuri, Nyla A. Heerema.
Internal Medicine, Ohio State University, Columbus, OH; Dana Farber Cancer Institute, Boston, MA; Duke University Medical Center, Durham, NC; Pathology, Ohio State University, Columbus, OH; Medicine, University of Chicago, Chicago, IL.
Several prognostic factors in CLL including un-mutated VH mutational status, select interphase cytogenetic abnormalities [del(11q22.3), del(17p13.1)], and p53 mutations have been associated with decreased time from diagnosis to symptomatic disease requiring treatment as well as shortened progression-free survival (PFS) and overall survival (OS). To date, the impacts of these prognostic factors on treatment outcome with fludarabine and rituximab combination treatments have not been explored. The Cancer and Leukemia Group B recently reported results of a randomized phase II study (CALGB 9712) that added rituximab concurrently or sequentially to fludarabine as initial therapy for symptomatic, untreated CLL (Blood 2026; 101:6). After a recently updated median follow-up of 43 months, PFS and OS are still similar in both arms. Of the 104 patients enrolled, we studied 88 patients for whom pre-treatment samples were available to examine the impact of VH mutational status (>97% defined as un-mutated), common cytogenetic abnormalities, and p53 mutational status on outcome relative to CR (complete remission) and PFS. OS was not examined because only 13 deaths have occurred to date. A total of 46 out of 75 (61%) patients were VH un-mutated CLL. Fifteen (52%) of 29 mutated and 20 (43%) of 46 un-mutated patients achieved a CR (p= 0.49). The median PFS among the VH mutated patients was 46 months [95% CI (40, 54)] whereas for un-mutated it was 32 months [95% CI (22, 42)] (p= 0.05). Controlling for differences in age, sex, WBC, LDH, and stage resulted in an adjusted p-value of p=0.03. Only four patients had p53 mutations, preventing analysis of this biologic feature independently. Using the Dohner hierarchical classification, the frequency CR rate and PFS for each group are summarized below.
Cytogenetic |
del(17p) |
del(11q) |
del(6q) |
tris12 |
del(13q) |
Normal |
Number pts. |
3 |
15 |
1 |
23 |
24 |
22 |
CR% |
0 |
53 |
100 |
25 |
38 |
34 |
PFS (months) |
18 |
25 |
42 |
42 |
45 |
49 |
Using the hierarchical classification of Dohner, there was not a difference in CR rate (p=0.25) and PFS (p=0.10). Controlling for differences in age, sex, WBC, LDH, and stage resulted in an adjusted p-value of p=0.04. The PFS for the del(17p13.1) and del(11q22.3) patients was significantly shorter than that observed for the remaining cytogenetic groups (p=0.03). We next sought to define a high-risk group of CLL patients, as having any of the following: VH un-mutated (>97%), del(17p), del (11q), or non-silent p53 mutation. Using this classification, 35 patients were assigned to the low-risk and 53 to the high-risk groups. The CR rate in each group was 43%. However, the median PFS among the low risk patients was 45 months with 95% CI (45, NA) whereas the median PFS among the high-risk patients was 32 months with 95% CI (22, 42) (p=0.004). These data demonstrate that high risk CLL patients characterized by VH un-mutated (>97%), del(17p), del (11q), or non-silent p53 mutation appear to have a shorter PFS with chemoimmunotherapy and define a subset of patients for whom additional novel treatment approaches should be targeted.
Abstract #476 appears in Blood, Volume 104, issue 11, November 16, 2026
______
See what I mean about the details being important? Fludarabine loses a lot of its glamour compared to the older chlorambucil, if one looks at the bigger picture. For the individual patient with specific FISH results, the breakdown of the RF therapy results into different risk stratified groups is a lot more useful than general results reported for a mixed bunch of patients.
This also highlights the point I was trying to make earlier: response rates and survival statistics can be easily skewed by the mere fact of how patients were recruited for a given clinical trial. If the patient cohort had an unusually high percentage of patients in early stage CLL, or proportionately more patients with 'good' cytogenetics were recruited, such "cherry-picking" can easily make the stats look a lot more rosy for that trial. Comparison of response statistics among clinical trials is meaningless unless the patient groups involved are well matched. And to judge that, nothing can replace the value of detailed and full-length papers published in peer-reviewed journals, where the results are subjected to scrutiny, researcher and design biases accounted for.
You know the old saying, trust but verify. Making therapy choices on the basis of less than adequate documentation of results that have not withstood the rigorous process of peer review and multi-center testing is tantamount to buying a pig in a poke. You may get lucky, and then again you may not. Our pop culture seems to have made rock-star status important in how we elect our public officials, but surely we can be a little bit more objective when it comes to choosing our therapy options! I realize we do not always have the luxury of waiting until all the information is available in this era of rapid change. All the more reason why we should hold the research community's feet to the fire and make sure they understand their ethical obligation to publish their results promptly. This is especially true if the results are less than expected, or there were more negative side effects. Clinical trial reports should not read like publicity releases, with as much "spin" as we have come to expect in politics!
A scandal that should make us sit up and take notice has received much attention in recent months. It seems many clinical trials funded by drug companies with vested interest report results only when they are positive, when they boost profits or the stock price for the company in question. Negative results are quietly deep-sixed, many of them never to see the light of day. This has become such a huge issue that a vast majority of the leading professional journals have recently signed a new pledge: in future papers will be accepted for publication only if the clinical trials they discuss have been formally announced in a public forum (such as www.clinicaltrials.gov) so that all patients have access to them, and even more importantly, the researchers took their responsibility seriously for prompt, detailed and even-handed publication of the results. Here are a few links if you want to read more about this issue, I have chosen a selection of them for your reading pleasure, from the New England Journal of Medicine, the PhRMA member companies representing the drug industry, and the Boston Globe.
NEJM Article;
PhRMA Clinical Trial Registration Proposal;
Boston.com: AMA calls for total drug trial disclosure
Patients who volunteer to take part in clinical trials risk their very lives in a good-faith effort to contribute to improving our knowledge, in the hope that their generous efforts will help make things just a bit easier for future generations. The researchers who use human subjects in clinical trials have a moral and ethical obligation to respect their wishes. Soon after the second world war, when horrific use of human beings with no rights used as experimental subjects in bizarre clinical experiments came to light, the great majority of nations signed the Declarations of Helsinki as a way of codifying the rights of human subjects in clinical trials. Principle 27 of the Declaration of Helsinki, spells out the need for public dissemination of trial results. This is not a trivial issue. No reputable institution or researcher should consider themselves above scrutiny, above accountability. It is not a matter of lack of 'respect' and 'trust', nor should our insistence upon prompt and detailed publication of clinical trial results be a cause for taking offense. Gentlemen, accountability goes with the territory of being clinical researchers dealing with human subjects.
http://www.assert-statement.org/publication.html
Publication of Results of Clinical Trials
Failure to report research results, positive or negative, in a timely manner adversely affects the relevance of published results of other trials and the utility of Systematic Reviews and Meta-analyses of trials (publication bias). Patients who agree to participate in clinical research, including clinical trials are primarily motivated by three factors: the anticipation of health-related benefits, and an expression of trust in the physician proposing research participation, and an altruistic hope that future patients will benefit from the knowledge gained. Failure to publish the research results dishonors such principled volunteerism. The research participants, whose enrollment made the clinical trial possible, are entitled to know the results of the trial, and the implications for their health.
The duty to share new knowledge with colleagues is a longstanding norm in the medical profession, commonly enunciated in professional codes of conduct. This imperative applies also to the acquisition of knowledge by a physician engaged as an investigator in clinical trials. Full publication of results in peer-reviewed print and online journals should be the primary means of results dissemination. Results of clinical trials are often presented initially in the form of abstracts or short reports at medical conferences. The utility of such reports is limited, particularly with respect to an assessment of the trial's internal and external validity, and the inability to incorporate the results of these trials in Systematic Reviews or Meta-analyses of the pertinent clinical literature. Thus, only full reports of trials should be considered an acceptable form of results dissemination.
Research ethics committees typically have oversight obligations during the ongoing conduct of research- related activities. For example, in the U.S., federal regulations specify the need for continuing review and re-approval of research at intervals not exceeding one year. Failure to report research results should be considered a form of research misconduct. Such failure is a proper consideration when the investigators submit new research proposals for review. Unfortunately, such considerations are necessary in the current research environment wherein financial conflicts of interest and questionable industry-investigator alliances not uncommonly culminate in attempts to suppress the dissemination of unfavorable research results.
 Enter Keywords: |
———
Disclaimer: The content of this website is intended for information only and is NOT meant to be medical advice. Please be sure to consult and follow the advice of your doctors on all medical matters.
Copyright Notice:
Copyright © 2026-2007 CLL Topics, Inc. All Rights Reserved.
All materials contained on this site are protected by United States copyright law and may not be reproduced, distributed, transmitted, displayed, published or broadcast without the prior written permission of CLL Topics, Inc. You may not alter or remove any trademark, copyright or other notice from copies of the content.
However, you may download and print material from CLLTopics.org exclusively for your personal, noncommercial use.
———
