|
||||||||||
Date: August 11, 2026
by Chaya Venkat
Related Articles:
As you can imagine, I have been doing my due diligence and learning all about cord blood transplants for CLL patients. The plans of my favorite hypothetical patient, Harvey, depend on the availability of this technology (for details see Harvey's Battle Plans). I would like to share with you the results of my research. It is quickly becoming clear to me that many more patients will be looking at this technology than I imagined. A lot has been learned in the past few years, and the technology has come up the learning curve rapidly.
The present day orthodoxy defines the best source of stem cells for transplanting in adult patients is a matched sibling donor, followed by a matched unrelated donor, and lastly stem cells from cord blood. The first leg of this sequence is already becoming not quite a slam dunk. Recent large scale mini-allo transplant studies from the Hutch (The Only Real Cure) suggest that matched unrelated donor transplants may be more effective than sibling donor transplants. Perhaps it has to do with the fact that grafts from unrelated donors are more likely to have a strong response when they encounter CLL cells in your body, and go after the cancer cells with a vengeance. In other words, nepotism is never a good idea when you want to get the job done right and stem cells from siblings may be a bit more “lazy” in mounting a full scale graft-versus-leukemia (GVL) effect. The relapse rates seem to be higher with sibling donor grafts than unrelated donor grafts, based on the data from Hutch.
There are other reasons why sibling donor grafts may be an issue, particularly in CLL. I have heard of at least a few anecdotal cases where the loving sibling is a perfect match, able and willing to donate stem cells to save the life of a beloved brother or sister. But come time to do a full scale work-up, it turned out the sibling had incipient and undetected CLL as well, and was therefore ineligible to be a donor! This is not as odd as you would think, given the well understood familial nature of CLL (Not the Worst Day of Your Life).
|
Number |
Probability |
|
One |
25% |
|
Two |
44% |
|
Three |
58% |
|
Four |
68% |
|
Five |
76% |
|
Six |
82% |
|
Seven |
87% |
|
Eight |
90% |
|
Nine |
92% |
|
Ten |
94% |
Another obvious issue is that CLL patients looking for a transplant are likely to be older, especially now that they are transplanting patients into their seventies and even older, thanks to the advent of “mini-allo” transplants. With older patients, their siblings are also not likely to be spring chickens themselves. I have not seen any definitive data to support this, but it seems to me that having a young donor in roaring good health is an advantage all around, both for the donor and the recipient of the stem cells. There is no getting away from the fact that as we age, we are likely to pick up more health issues. That is equally valid for a potential sibling donor as well: he or she is also likely to be getting a little long in the tooth.
Not everyone has the luxury of a matched sibling donor. Let’s face it, family sizes are shrinking across the board. Just a generation ago, large families with several kids per household (with the same two parents) were the norm. Now we have smaller families, sometimes shorter-term families — and often the kids can be parsed into his kids, her kids and their kids. As far as genetics goes, the chances of getting a full match from a half-brother or half-sister are much lower than from a full sibling. Your chances of having at least one fully matched sibling increase as the number of siblings increase, getting close to a certainty (but not quite) if you happen to have 10 siblings.
As you can see there will be many patients who do not have the luxury of a good sibling match and if they are among population minorities, their chances of finding an unrelated donor is slim to none. As an added complication, matching criteria have become more precise. Just a few years ago, a good match was 8 out of 8 HLA markers. Then it became 10 out of 10. Now I understand some of the centers are looking for 12/12 matches. All of this means that as we get more picky about what constitutes a good match, it is going to be harder and harder to find good matches among adult donors, especially if you are not of cookie cutter ethnicity.
Ethnicity |
Probability |
|
Caucasian |
52.4% |
|
African American |
8% |
|
Asian/Pacific Islander |
6.5% |
|
Hispanic |
8.5% |
|
Native American |
1.2% |
|
Multi-Racial |
2.2% |
|
Unknown |
21.1% |
The take home lesson is that not every CLL patient who wants to undergo and happens to qualify for a stem cell transplant will have access to a sibling donor or a well matched unrelated donor. Prior to the development of cord blood transplants, these guys were just plain out of luck. Let me be blunt about this: up to now, the only therapy of proven curative potential for CLL is a stem cell transplant from a healthy donor. Neither alphabet soup combinations of chemo-immunotherapy drugs nor autologous transplants can claim to actually CURE our disease. The remissions may be long, the remissions may be deep, depending on the nature of your disease and the oomph of the chemo combination, not to mention a certain amount of luck. But eventually, sooner or later, our experience to date has been that all of these remissions will relapse and the darn disease will raise its ugly head again. Down the road, this may change. I will be keeping my fingers crossed for a truly curative therapy for CLL, one that is going to get this monkey off of our backs without causing so much harm in the process that it kills the patients as well. But for now, allogeneic transplants are the only therapy that can achieve a full cure of CLL. Not having access to this life saving technology was a huge issue for many patients.
As we discussed above, the obvious advantage of cord blood as a source of stem cells for transplanting is its improved availability. A recent review of the transplant scene by Wagner, et al., (the first abstract below) states that roughly a third of patients who need a stem cell transplant have a suitable sibling donor. Another third can find a matched unrelated donor in the 10 million or so donors registered in donor banks around the world. For the remaining third, especially if they are from racial and ethnic minorities, cord blood transplants constitute a welcome life-line.
Timing is everything. Think about this: you have CLL, you have been through several rounds of therapy, and you have just failed your last choice of chemo sooner than you hoped, sooner than you expected. Your best choice is a mini-allo transplant – but your parents were not thoughtful way back when and you have no brothers or sisters – at least none that match for a transplant. How long will it take you to find a matched unrelated donor?
Obviously, that depends on your gene type, your racial and ethnic persuasion. But even for you folks with standard issue genetics, the sheer process of searching, locating and confirming a suitable adult donor will take anywhere between 3-4 months if you are lucky, a lot longer if you are not. That could be an excruciating wait, while you watch your CLL gradually grow bigger and meaner.
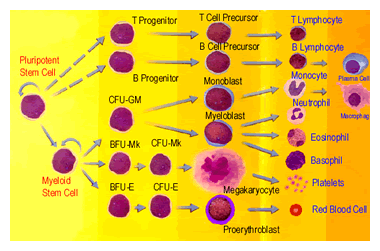
Cord blood is collected from the umbilical cord and placenta after baby is born. Prior to our appreciation of the tremendous value of this precious material, this was routinely discarded as waste. Since the cord blood is collected after the baby is born, its collection poses no inconvenience or danger to the mother or child. Cord blood is processed, well documented at the time of collection and then frozen for later use. Once a unit is identified as suitable for a given patient (by means of an electronic data base search), it is immediately available for use. Roughly 400,000 units of cord blood units are presently available in donor banks, and the number is increasing over time, as we become aware of the value of this product that was once discarded in the trash. The typical waiting times are a matter of weeks for matching recipients with cord blood units, not months as with adult stem cell donors. Another advantage of cord blood is that it is far less likely to be positive for a variety of viruses such as CMV (cytomegalovirus) that are routinely present in adult donor grafts.
One of the reasons why it is so hard to match adult donors with patients is that the adult donor has to match the patient very closely and this becomes even harder if we get picky and require a 12 out of 12 HLA match. If the matching is not quite as good as we would like (say a couple of the HLA markers don’t quite match) there is an increased risk that the graft may not take (it may be rejected by your body) or that it will cause extensive graft-versus-host disease (“GVHD”: this is where the newly grafted immune system thinks your body is the enemy and starts attacking your tissues indiscriminately — see GVHD). Things have to be exactly so for the host (you) and the graft (from the adult donor) to live in peaceful co-existence, and that makes the matching process tricky.
Stem cells from cord blood seem to be more accommodating. I can go into the details of why this is so (such as cord blood has more regulatory cells controlling unwanted over-the-top immune responses), but here is a more layperson view of it that works for me. Think of the baby co-existing within the mother’s womb for the nine months of pregnancy. If the baby’s cord blood is too finicky and takes exception to the differences between it and the mother, that would not bode well for the pregnancy, would it? Nine months of two different individuals with different genetic patterns living together in such close contact gives us the clue – cord blood by its very nature has to be more adaptable, able to get along better with slightly mismatched surroundings without throwing a hissy fit.
As it turns out, it is necessary to match cord blood to the patient on only 6 HLA markers. While it is very nice to get a 6-out-of-6 matched cord blood unit, even that level of matching is not mandatory for a successful transplant. Cord blood transplants are routinely done with matching as low as 4 out of 6 HLA markers, without running into unacceptable problems.
This flexibility is the huge advantage of cord blood as a source of stem cells for transplants. It means that the vast majority of patients who need a transplant but cannot find suitable adult donors can still find a well matched cord blood source. People like our hypothetical friend “Harvey” would have been up the proverbial creek without a paddle, but for this technological breakthrough. “Harvey” had no sibling or unrelated adult donors that matched him sufficiently. But he does have several well matched cord blood units in the banks! Trust me, I sighed a breath of relief when I got that bit of news.
Cord blood has been used as transplant material for treating children for a lot longer – transplanting adults with this source of stem cells is the new development. This brings up an important difference between children and adults. We are a lot bigger. The stem cell dose available in the umbilical cord and placenta from a baby is plenty large for treating children, cord blood is just chock full of stem cells. But the number of available stem cells becomes a real issue when we consider an adult weighing many more kilograms. Much of the new and exciting research in the area of cord blood transplants for adult patients focuses on this issue: how to get around the problem of the small number of stem cells when we are talking about adult recipients, how to get more stem cells per kilogram of body weight. I will describe some of these new developments in this review.
In the early years of cord blood transplants in adult patients, the importance of stem cell dose was not quite appreciated. Many patients who received a single cord blood unit for transplant got too few stem cells for their body size, and this resulted in significantly higher risk of graft failure because the new stem cells coming in failed to establish a foothold in their bone marrow, grow and flourish to become a full fledged immune system. Or, it took way too long for the few stem cells to get established sufficiently and quickly generate a sufficient number of white blood cells to protect against infections. Delayed engraftment and production of neutrophils from the new graft means patients were at risk of contracting life-threatening opportunistic infections during this period of vulnerability and this added to the mortality in these early attempts. This was the single biggest roadblock, the single biggest negative associated with cord blood transplants for adults, compared to using stem cells from adult donors.
The solution pioneered by researchers at the University of Minnesota was quite logical and simple. If one unit of cord blood is not enough to do the job, why not use two of them? This is exactly what they did and it has just about revolutionized the whole process. More than a hundred adult patients have now been transplanted with two cord blood units. It is important that both the cords are reasonably matched to the patient, and both cords also matched with each other. Interestingly, when the dust settles, only one of the two cords transplanted wins and the loser gradually fades away over time. But in the crucial early days immediately after transplant, doubling up on stem cell dose by using two cord blood units makes all the difference. Transplant failure rates plummeted, engraftment happened sooner, patients got life preserving immune protection sooner, mortality due to infections started coming down. Talk about a steep learning curve!
Below is the abstract of a detailed and comprehensive review of the state of the art double cord transplantation in adult patients. Do write to us if you want to read the full text of this important review.
Blood. 2026 Jun 14;
Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplant outcomes in 110 adults with hematological disease.
Brunstein CG, Barker JN, Weisdorf DJ, Defor TE, Miller JS, Blazar BR, McGlave PB, Wagner JE.
Division of Medical Hematology, Oncology & Transplantation, Blood and Marrow Transplant Program, University of Minnesota Medical School, Minneapolis, MN.
We evaluated the efficacy of UCB in the setting of a nonmyeloablative regimen consisting of fludarabine (200 mg/m(2)), cyclophosphamide (50 mg/kg) and a single fraction of total body irradiation (200 cGy) with cyclosporine and mycophenolate mofetil for posttransplantation immunoprophylaxis. The target cell dose for the UCB graft was 3.0 x 10(7) nucleated cells/kg resulting in the selection of a second partially HLA matched UCB unit in 85%. One-hundred and ten patients (median age: 51 years) with hematological disease were enrolled. Neutrophil recovery was achieved in 92% at median of 12 days. Incidences of grades III-IV acute and chronic graft-versus-host disease (GVHD) were 22% and 23%, respectively. Transplant-related mortality was 26% at 3 years. Survival and event-free survival (EFS) at 3 years were 45% and 38%, respectively. Favorable risk factors were absence of high risk clinical features (Karnofsky 50-60, serious organ dysfunction, recent fungal infection, p<0.01) and absence of severe GVHD (p=0.04) for survival, and absence of high risk clinical features ( p<0.01) and use of two UCB units (p=0.07) for EFS. These findings support the use of UCB after a nonmyeloablative conditioning as a strategy for extending the availability of transplant therapy particularly for older patients.
PMID: 17569820
____________
What are the patient characteristics for successful transplantation, with reduced mortality risk? Here are some points to ponder:
This is the holy grail of cord blood transplant science. Several transplant centers are looking at ways of expanding the number of stem cells present in the cord blood unit. The Fred Hutchinson Cancer Research Center in Seattle is looking at expanding the stem cell numbers in the lab (“ex-vivo”, meaning outside the body) by manipulating the cord blood with a careful mixture of growth factors and signaling molecules. They (the Hutch) have a clinical trial going on right now, where patients are given one “unmanipulated” unit of cord blood, and another unit that has gone through expansion efforts in the lab.
Another interesting approach pioneered by Dana-Farber is being considered in a multi-center trial (Dana Farber, M. D. Anderson and the Hutch). This approach is trying to increase stem cell counts within the patient’s body (“in-vivo”), soon after the cord blood is infused. They are using a new man-made version of parathyroid hormone (PTH) to achieve this. PTH has been recently approved for treating osteoporosis, mostly in post-menopausal women. Several thousand women have been on this drug for more than a couple of years, with good improvement in bone density and little or no adverse effects. The interesting thing is that in the process of improving bone density, the bone marrow niche seems to become more hospitable to the health and welfare of stem cells, allowing them to increase in numbers – sort of like throwing a welcome-to-the-neighborhood-party to make the new stem cells feel at home in your bone marrow, encouraging them to multiply.
The Hutch approach (ex-vivo) and the Dana Farber approach (in-vivo) are both attempting to do the same thing, tickle the stem cells to multiply by activating something called the Notch pathway. This is not an easy thing to do. Stem cells are finicky by nature, and if you “goose” them too hard, they will divide into two cells of committed lineage. In other words, if pushed too hard they tend to lose their special talents as stem cells, their ability to grow many different types of blood cells. When stem cells lose their “stem-ness”, they are far less valuable. Instead of a multi-talented stem cell, we might end up with two progenitor cells with limited repertoire, cells that can only produce one kind of blood cell. In keeping with my preferred belt-and-suspenders attitude to life, both the ex-vivo and in-vivo approaches use double cords for transplanting.
These are early days for the stem cell expansion projects, and only time will tell how things play out in the long run. Both the Hutch and Dana-Farber clinical trials are presently restricted to patients under the age of 45, since both are full myeloablative transplants and older patients do no do very well in this aggressive conditioning regimen. But I am told that both approaches will be opening mini-allo versions of their protocols in the near future, as early as the first quarter of 2026, allowing us older folks to have access to them. Below are some links and an interesting abstract, if you wish to learn more about these cutting edge research efforts.
Expanding Cord Blood Stem Cell Numbers in the Lab at the Hutch;
Phase II/III Clinical Trial Listing for Ex-vivo Expansion Technology: NCT00469729.
Bone Marrow Transplant. 2026 Jun;39(11):655-60.
Targeting the stem cell niche: squeezing blood from bones.
Ballen K.
Division of Hematology/Oncology, Massachusetts General Hospital, Boston, MA 02214.
During human development, stem cells establish themselves in specific anatomic locations or niches. The niche harbors the stem cells, and regulates how stem cells proliferate. The interaction between stem cells and their niche affects stem cell function, and offers an opportunity to improve the marrow microenvironment. Osteoblasts produce hematopoietic growth factors and are activated by parathyroid hormone (PTH). A calcium sensing receptor, expressed by hematopoietic stem cells, regulates the niche and can be targeted to increase stem cell numbers. Therefore, drugs that affect osteoblast function or target calcium receptors may be useful for stem cell mobilization and engraftment. In this review, the biology of the stem cell niche and the potential therapeutic manipulations of the stem cell niche are reviewed. PTH is in clinical trials for patients who have not mobilized autologous stem cells well. The limiting cell numbers for adult cord blood transplantation increase the risk of infection, and PTH is currently in a clinical trial following cord blood transplantation in an effort to improve engraftment and immune reconstitution.
PMID: 17401397 [PubMed - indexed for MEDLINE]
____________
There are many for-profit outfits out there that will offer to save your baby’s cord blood for you, for a hefty fee. Should you do it?
I strongly urge you not to fall for the sales pitch. For starters, there is no way of telling if the company in question would do a good job of preserving the cord blood, whether they would still be around decades later, whether they will pay their electricity bills promptly so that the refrigeration does not get turned off. Second, chances are good your child (or anyone in your family) will never need the cord blood anyway. Third, even under correct cryo-preservation conditions, cord blood could decay over the decades it sits in the freezer. Why would you want to use a source of stem cells that may have gotten just a little too “ripe” waiting around frozen for decades? Last but not least, with the exception of the baby itself, the cord blood you save will not be a perfect match for anyone else in your family, and you are far more likely to get a better match out in the public donor banks, if and when you need it. For us who are going to be proud grandparents, cord blood from your brand new grandkid would have genetic contribution from four individuals (both parents and their parents). In other words, your genetic contribution is limited to only one out of four.
So, please, do not waste your money and the precious resource by trying to save your baby’s cord blood in personal storage banks. Do the right thing. Donate your baby’s cord blood to a public cord blood bank, and encourage all soon-to-be mothers you know to do the same. What goes around comes around. Increasing the number of cord blood units in public donor banks will help each of us, when and if the need arises. And the baby's own life will start with the wonderful gift of life to another human being.
 Enter Keywords: |
———
Disclaimer: The content of this website is intended for information only and is NOT meant to be medical advice. Please be sure to consult and follow the advice of your doctors on all medical matters.
Copyright Notice:
Copyright © 2026-2007 CLL Topics, Inc. All Rights Reserved.
All materials contained on this site are protected by United States copyright law and may not be reproduced, distributed, transmitted, displayed, published or broadcast without the prior written permission of CLL Topics, Inc. You may not alter or remove any trademark, copyright or other notice from copies of the content.
However, you may download and print material from CLLTopics.org exclusively for your personal, noncommercial use.
———