 |
||||||||||
Date: March 18, 2026
by Chaya Venkat
Articles on Therapy Choices
The reviews we publish on this website are almost always based on abstracts and learned papers from acknowledged experts and precious wisdom gleaned from prestigious journals. I strayed from our normal reporting style in a recent article (Winning the Battle but Losing the War?), which was almost entirely editorial comments. It is fun being a “cancer guru” on a rare occasion, but I think it is time for me to get back to my regular role of layperson reporter. This review deals with a pivotal paper that was just published in Blood. It attempts to answer a simple question: does the combination of fludarabine + cyclophosphamide do a better job than just fludarabine as single agent? Do we get more bang for the buck by combining the killing powers of the purine analog (fludarabine) with the alkylating agent (cyclophosphamide)? Do the laws of arithmetic work in this case, is 1 + 1 = 2? I think you will be surprised by the answer. Welcome to the surreal world of cancer math.
With a little bit of extrapolation, the lessons learned from this paper may also give us a perspective on a similar and important question: Is RFC (Rituxan, fludarabine, cyclophosphamide) better than RF, as a result of the extra “C” added to the mix? These two combos, RFC pioneered at M. D. Anderson and RF pioneered at Ohio State, are the two lead candidates for Top Gun award right now in CLL. Unfortunately it will be a while before we see honest apples-to-apples comparison of these two combinations in well designed, randomized, multi-center phase-3 clinical trials. There is way too much bias in single institution phase-2 studies and any comparison of response statistics is an "apples to kumquats" comparison at best. But perhaps if we understand the F+C versus F comparison, maybe the same logic will help us get a better handle on comparing RFC and RF.
If you follow the logic for treating solid cancers described in our recent editorial (Winning the Battle), it seems obvious that we will get more killing power as we add more bells and whistles. The logic works like this: if we give the patient just enough of each drug so that it is below the “maximum tolerated dose”, but we increase the alphabet soup by adding more drugs to the menu, we will be able to kill the cancer but not kill the patient at the same time. No kidding, this logic has stood the test of time in treating a variety of cancers, mostly solid cancers. Often enough they add radiation and surgery to boot. The words “Shock and Awe” come to mind.
 |
Until recently, fludarabine (“Fludara”) was the de-facto “gold standard” for treating CLL, even though there have been some vocal dissenters who believe it does not give any survival advantage over the more old fashioned chlorambucil (Shopping for Therapies). Cyclophosphamide (“Cytoxan”) is an alkylating agent, similar to chlorambucil (“Leukeran”). Surely it makes sense to combine fludarabine and cyclophosphamide to get the CLL cells right in the cross-hairs? Or does it? Would it be better to add even more drugs to the agenda? Just look at the diagram on the left, it seems to suggest a definite advantage to a longer string of drugs. But I suggest you don’t quit on me at this stage, please do make the effort to read the rest of the article. It is worth your time, in my opinion.
The results of an excellent Phase 3 German clinical trial were reported just last month, where this combination of fludarabine + cyclophosphamide was compared with single agent fludarabine. This was a large study — a total of 328 previously untreated patients participated in it. They were randomly assigned to either the F or F+C arms of the trial, well matched across a host of criteria. The trial was conducted over many centers. Randomized phase three trials such as this are very valuable since they make it possible to compare apples to apples. Randomization of patients to the two arms reduces risk of “cherry picking”, and the multi-center design makes it harder for researcher or institution bias to skew the results. Unfortunately, rigorous studies such as this are going out of style. They cost too much, they take too long, and they do not lend themselves to easy sound bites or press releases. But since our very lives depend on making the right therapy choices, I think you and I will take the time to wade through this detailed paper. The abstract is given below. Write to us if you want help in locating the full text of the article or the Kay editorial that accompanied it.
Blood. 2026 Feb 1;107(3):885-91. Epub 2026 Oct 11.
Fludarabine plus cyclophosphamide versus fludarabine alone in first-line therapy of younger patients with chronic lymphocytic leukemia.
Eichhorst BF, Busch R, Hopfinger G, Pasold R, Hensel M, Steinbrecher C, Siehl S, Jager U, Bergmann M, Stilgenbauer S, Schweighofer C, Wendtner CM, Dohner H, Brittinger G, Emmerich B, Hallek M; German CLL Study Group.
Department of Internal Medicine I, University of Cologne, Kerpener Str 62, D-50924 Koln, Germany.
Combination chemotherapy with fludarabine plus cyclophosphamide (FC) was compared with the standard regimen of fludarabine monotherapy in first-line treatment of younger patients with chronic lymphocytic leukemia (CLL). Between 1999 and 2026, a total of 375 patients younger than 66 years who predominantly had advanced CLL were randomly assigned to receive either fludarabine (25 mg/m(2) for 5 days intravenously, repeated every 28 days) or FC combination therapy (fludarabine 30 mg/m(2) plus cyclophosphamide 250 mg/m(2) for 3 days intravenously, repeated every 28 days). Both regimens were administered to a maximum of 6 courses. FC combination chemotherapy resulted in significantly higher complete remission rate (24%) and overall response rate (94%) compared with fludarabine alone (7% and 83%; P < .001 and P = .001). FC treatment also resulted in longer median progression-free survival (48 vs 20 months; P = .001) and longer treatment-free survival (37 vs 25 months; P < .001). Thus far, no difference in median overall survival has been observed. FC caused significantly more thrombocytopenia and leukocytopenia but did not increase the number of severe infections. In summary, first-line treatment with FC increases the response rates and the treatment-free interval in younger patients with advanced CLL.
PMID: 16219797
____________
All of the patients recruited for this study were chemo naïve and relatively young patients, but with progressing CLL that needed therapy. As you can see from the table below, the randomization of the patients between the two groups was as good as it gets, the miracle of computerized randomization. This is the advantage of large studies such as this one, the statistics are robust and the results can be trusted. The full text of the paper has the breakdown of the two patient groups in greater detail, if you are interested. I have also changed the units to more familiar American units (my apologies to our international readers).
Characteristics |
F |
FC |
|---|---|---|
| Number of Patients | 182 |
180 |
| Median Age (years) | 59 |
58 |
| Rai Stage (%) | ||
| 0 | 2.4 |
3.1 |
| 1 or 2 | 56.6 |
58.2 |
| 3 | 41.0 |
38.7 |
| Median ALC | 70.4 |
65.8 |
| Median Hemoglobin | 12.7 |
13.2 |
| Median Platelet Count | 148 |
145 |
| Median B2M | 2.75 |
2.75 |
This study demonstrated a higher percent of patients responded to the combination of fludarabine + cyclophosphamide than they did to fludarabine alone, and more of them got “Complete Responses” by the NCI definition of the term.
Response (%) |
F |
FC |
|---|---|---|
| Complete Response (NCI) | 6.7 |
23.8 |
| Partial Response | 76.2 |
70.7 |
| Overall Response | 82.9 |
94.5 |
So far so good, the “Complete Response” and “Overall Response” for the F+C combination are statistically significant and better. The definition of “CR” followed the NCI criteria established quite a while back, based on physical examination of the patient, blood counts, and the presence of less than 30% lymphocytes in the bone marrow upon biopsy. These guidelines were established prior to routine use of imaging technology such as CAT scans. Our intrepid researchers went the extra mile and used imaging to define the status of their patients. Here is what happened to the percentage of CRs, when they looked a bit more closely for remains of cancer in their patients.
Response (%) |
F |
FC |
|---|---|---|
| Complete Response (Imaging) | 4.9 |
16.5 |
| Partial Response | 78.0 |
78.0 |
| Overall Response | 82.9 |
94.5 |
Notice the big hit taken by the percentage of CRs in both cases! A significant number of the CRs got down graded to mere PRs when CAT scans were used. I guess the definition of a “Complete Response” depends on how closely one looks at it. As we get more sophisticated in looking for cancer cells, many of the CR statistics reported in older articles will fall by the wayside. That is not a guess on my part, it is a fact. This impressive study was accompanied by an equally thought provoking editorial by Dr. Neil Kay of the Mayo Clinic. This is what he had to say about it:
“This trial has illustrated several important issues… the use of large randomized phase 3 trials such as this study underscore that overall response rates and CR levels seen in phase 2 trials are influenced by patient selection and are often higher than those observed in large phase 3 studies.
Of most importance, they have reported that with the use of computed tomography scans and ultrasound a significant downstaging of CR rates occurs particularly for the FC. This outcome should give clinicians pause when telling patients that they are in CR and strongly endorses the need for updating the NCI 96 standards used to determine levels of clinical response in CLL.”
Write to us if you want to read Dr. Kay’s full text editorial. And the next time someone “guarantees” you a “CR” (let alone a cure!) if you opt for his/her pet therapy combo, you might want to ask for a more detailed definition of that promised “Complete Response”.
There is one additional snippet on the subject of response statistics. While there was a statistically significant difference between the CRs and ORs between single agent F versus F+C when we looked at the whole patients cohort, much of the advantage of F+C was limited to late stage patients only. When the data is sliced to reflect early, medium and late stage patients (according to their Binet stages A, B and C), there was no difference (statistically speaking) between the responses obtained by using F versus F+C for Binet Stage A patients. Moral of the story — for early stage patients, there was not much to be gained by taking on the extra toxicity of F+C since they would have done just as well with single agent F.
Overall Response (%) |
F |
FC |
|---|---|---|
Stage A |
89.5 |
92.3 |
Stage B |
85.4 |
93.9 |
Stage C |
76.8 |
96.2 |
It is all very nice to collect response statistics immediately after the end of therapy. It might even give you a good feeling, temporarily, when you find that you are in the coveted “Complete Response” group after having gone through the rigors of therapy. But if you are like me, that is but temporary gratification — the appetizer before the real meal. The million dollar question is this: will patients live longer because they got F+C, compared to single agent F? The graph below was a big eye-opener for me on that front.
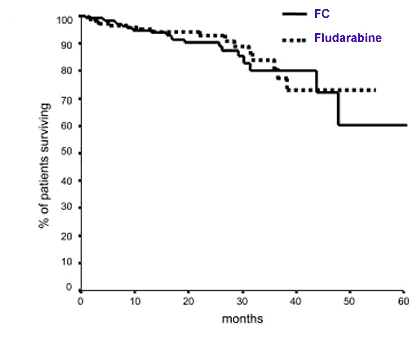
Survival after FC or F
If you are not familiar with reading these graphs, let me make it easier for you. Over the 60-odd months over which the two groups of patients were followed, roughly the same percent of patients died in each group! It would have been small comfort to the families of the patients who died that the F+C group had better response statistics immediately after completion of therapy!
As we go shopping for therapies, we have some pretty basic needs:
So, using this list, how does F+C compare with single agent F?
Criterion |
F |
FC |
|---|---|---|
| Toxicity during therapy? | Less toxic |
More toxic |
| Cytopenia? | Less likely |
More likely |
| Chance of getting CR? | Lower |
Higher |
| Quality of Remission? | ?* |
?* |
| Length of Remission? | Shorter |
Longer |
| Length of Overall Life? | Same |
Same |
That last item — for me that is the one that trumps the rest of the issues combined! If you are partial to living as long as you can, this one item is the deal breaker. Maybe the two curves will separate out later on, but over the 60 months that these two groups were monitored, there was no advantage in the combination of FC over simple F, on this important yardstick.
* I used “?” on the quality of remission issue since this particular study did not dwell on it. However, a previous article from no less than Dr. Kanti Rai (of the Rai staging system fame) had this to say about combination of purine analogs (such as fludarabine) with alkylating agents (such as cyclophosphamide and chlorambucil):
J Clin Oncol. 2026 Sep 15;20(18):3878-84.
Therapy-related myeloid leukemias are observed in patients with chronic lymphocytic leukemia after treatment with fludarabine and chlorambucil: results of an intergroup study, cancer and leukemia group B 9011.
Morrison VA, Rai KR, Peterson BL, Kolitz JE, Elias L, Appelbaum FR, Hines JD, Shepherd L, Larson RA, Schiffer CA.
Section of Hematology/Oncology, Veterans Affairs Medical Center, Minneapolis, MN 55417, USA.
PURPOSE: Patients with chronic lymphocytic leukemia (CLL) may have disease transformation to non-Hodgkin's lymphoma or prolymphocytic leukemia; however, development of therapy-related acute myeloid leukemia (t-AML) is unusual. A series of patients enrolled onto an intergroup CLL trial were examined for this complication.
PATIENTS AND METHODS: A total of 544 previously untreated B-cell CLL patients were enrolled onto a randomized intergroup study comparing treatment with chlorambucil, fludarabine, or fludarabine plus chlorambucil. Case report forms from 521 patients were reviewed for t-AML.
RESULTS: With a median follow-up of 4.2 years, six patients (1.2%) to date have developed therapy-related myelodysplastic syndrome (t-MDS; n = 3), t-AML (n = 2), or t-MDS evolving to t-AML (n = 1), from 27 to 53 months (median, 34 months) after study entry. This included five (3.5%) of 142 patients treated with fludarabine plus chlorambucil and one (0.5%) of 188 receiving fludarabine; no chlorambucil-treated patients developed t-MDS or t-AML (P =.007). At study entry, the median age among these six patients was 56 years (range, 44 to 72 years); three were male; the CLL Rai stage was I/II (n = 4) or III/IV (n = 2). Response to CLL therapy was complete (n = 4) or partial remission (n = 1) and stable disease (n = 1). Marrow cytogenetics, obtained in three of six cases at diagnosis of t-MDS or t-AML, were complex, with abnormalities in either or both chromosomes 5 and 7. Other abnormalities involved chromosomes X, 1, 8, 12, 17, and 19. Median survival after diagnosis of t-MDS/AML was 3.5 months (range, 0.5 to 10.1 months).
CONCLUSION: Our findings raise the possibility that alkylator-purine analog combination therapy may increase the risk of therapy-related myeloid malignancies, which is of particular relevance with regard to ongoing trials using these combination therapies.
PMID: 12228208
____________
I think you would agree, running the risk of therapy related myeloid cancers would put a serious crimp on our quality of life issue.
One particular development strikes fear into the hearts of all CLL patients, and that is the risk of Richter’s Transformation. This transformation into a variety of large cell lymphoma is bad news. Most patients who are unfortunate enough to get this transformation can count their life span in months and not years. Used to be that anywhere from 3-5% of CLL patients had this transformation happen to them. But this historical percentage has begun to change in recent years. Some experts are beginning to see as many as 10-12% of CLL patients undergoing this transformation. There is an increasing consensus that this scary shift may be due to increasingly aggressive and immunosuppressive therapies used in treating CLL patients. Combinations such as F+C are more likely to cause broad spectrum loss of immune system defense. Lack of surveillance by neutrophils, T-cells and B-cells may allow viral infections (such as EBV cited below) to grow unchecked and this in turn may lead to an increased risk of Richter’s transformation and other secondary cancers. Do you know that most adults in the Western world have been exposed to Epstein-Barr virus, as well as Herpes virus? Once you are infected, these viruses are there forever in your body even after you recover from the initial infection and they can reactivate during a period when your immune defenses are down and out. Does F+C carry a higher risk of immune system dysfunction? Seems to be the case, since our German researchers did find higher incidence of cytopenia in the F+C arm of their trial. Does this translate to higher risk of viral reactivation? I would expect so, even though they did not notice any higher number of “serious” infections. Will there be a higher percent of secondary cancers and Richter’s transformations in the F+C group? Only time will tell.
Leuk Res. 2026 Apr;29(4):389-95.
Richter's transformation of chronic lymphocytic leukemia. The possible role of fludarabine and the Epstein-Barr virus in its pathogenesis.
Thornton PD, Bellas C, Santon A, Shah G, Pocock C, Wotherspoon AC, Matutes E, Catovsky D.
Section of Haemato-Oncology, Institute of Cancer Research, The Royal Marsden Hospital, Fulham Road, London SW3 6JJ, UK.
Transformation of CLL into a large cell lymphoma has an incidence of 3-5%. We have studied 101 cases of CLL treated with fludarabine over a 10-year period (1990-2000) and observed a 12% incidence of transformation. In six of 12 patients, transformation was documented within 4 months following treatment with fludarabine. Pathological material, available in nine cases, was investigated for latent EBV by staining for LMP-1 by immunohistochemistry and EBERs-1 and 2 by in situ hybridisation. LMP-1 and EBERs were demonstrated in three of the nine samples. In two cases there was a different pattern of immunoglobulin gene rearrangement in the transformed cells assessed by PCR (FR3 fragment) compared to the original CLL clone. One of these two cases showed evidence of latent EBV. The other seven cases, of which two were EBV positive, showed identical pattern of Ig gene rearrangement in both the CLL and the transformed cells. We suggest that the relatively high incidence of transformation in this series may be due to immunosuppression mainly related to fludarabine, although other agents and prior therapies may have also contributed.
PMID: 15725472
____________
While this rigorous and excellently done comparison between F+C versus F is interesting all by itself, it raises the possibility of another intriguing comparison. If F+C is not much better than F in prolonging life, and may indeed carry higher price tag in terms of toxicity and so forth, what is the rationale of using both F and C in combination with Rituxan? In other words, why use R+F+C, instead of R+F only, leaving out the C? Sure, there may be a slight edge in higher response statistics for RFC, but will the three drug combo lead to longer overall life? Will there be a price to pay for the higher response statistics in terms of a higher risk of secondary cancers and Richter’s Transformation? I expect this is something to think about, while you weigh the relative merits of RF versus RFC. In the absence of well designed multi-center phase-3 trials comparing these two front-runners, we have to make-do with semi-educated guesswork. If indeed a three drug combo is necessary, perhaps it might be a good idea to consider FCR Lite, a clinical trial we reviewed a few weeks ago, where the F+C component is downplayed.
 Enter Keywords: |
———
Disclaimer: The content of this website is intended for information only and is NOT meant to be medical advice. Please be sure to consult and follow the advice of your doctors on all medical matters.
Copyright Notice:
Copyright © 2026-2007 CLL Topics, Inc. All Rights Reserved.
All materials contained on this site are protected by United States copyright law and may not be reproduced, distributed, transmitted, displayed, published or broadcast without the prior written permission of CLL Topics, Inc. You may not alter or remove any trademark, copyright or other notice from copies of the content.
However, you may download and print material from CLLTopics.org exclusively for your personal, noncommercial use.
———
