 |
||||||||||
Date: January 8, 2026
by Chaya Venkat
Related Articles:
CLL Topics Sponsored Projects
Rituxan in the News

A while ago we published our review of an interesting article that described synergy between Rituxan and Fenretinide (Rituxan in the News). Fenretinide is an analog of vitamin A, developed by the National Cancer Institute. Another name for it is 4-HPR, short for 4-hydroxyphenylretinamide. I have been following this interesting molecule for a while now, and I am delighted to report that finally it is going to be in formal clinical trials for CLL patients. Read on, folks. This article may suggest an interesting therapy option to you that you were not aware of, until now. And there is ownership involved here as well. Part of this trial is paid for by your hard earned dollars, donated to CLL Topics!
I think most of us are familiar with the name Rituxan, and its ability to target CD20 carrying B-cells (Rituxan Therapy ). This monoclonal antibody has the tremendous advantage of being selective - it targets only mature B-cells, since they are the only cells that express the CD20 marker targeted by Rituxan. The not so wonderful news is that Rituxan does not do a very good job of killing CLL cells. In fact, single-agent Rituxan does not work very well in a majority of CLL patients and the remissions obtained are partial at best. On the other hand, it is now well known that combination of Rituxan with other drugs (such as fludarabine, pentostatin, cyclophosphamide, chlorambucil, etc.) works far better than either drug by itself. Combinations such as FR (fludarabine + Rituxan: see Fludarabine Monotherapy Is No Longer the Gold Standard and RF: Risks and Rewards) and FCR (F + R + cyclophosphamide: see FCR Lite) have become popular, and it has been demonstrated that very high response rates can be obtained by means of these chemo-immunotherapy combinations.
Here is the problem with all of the combinations proposed thus far of Rituxan with conventional chemotherapy drugs. In the process of improving response statistics, there is an undeniable increase in the toxicity of the regimens. You get a bigger bang but the price tag is steep as well, in terms of increased immune suppression, higher risk of opportunistic infections during the period of vulnerability and potential for secondary cancers due to the mutagenicity of the chemo. How then do you make therapy choices? When is the price tag worth the result? Tough call, as I am sure you would agree.
Me, I like to have my cake and eat it too. Hence my interest in combinations that keep the wonderful selectivity and relatively low toxicity of Rituxan, but combine it with an equally low toxicity second drug that is able to boost the cancer cell killing power of Rituxan. CLL Topics is proud to announce our sponsorship of a brand-new Phase II clinical trial that has just opened for recruitment at the University of Washington and the Fred Hutchinson Cancer Research Center, Seattle WA, designed to study the combination of Rituxan + fenretinide. This trial may well be that goldilocks scenario we have been looking for, a way of improving the efficacy of Rituxan without increasing toxicity. While the really big bucks needed to run the trial come from the NCI (these folks have much deeper pockets than us chickens), fully $20,000 of the price tag for this trial is paid for by your generous contributions to CLL Topics. Hey, I am all for leveraging our precious dollars!
This trial satisfies all of the criteria we have established as guidelines for our sponsorship (Our Sponsorship Philosophy).
In all sincerity, those of you who have donated money to support funding of this trial, and even more, those of you who volunteer for this clinical trial are the true heroes by my reckoning. Your generosity will pave the way toward developing better and cleaner therapy options for all the patients who will walk in your footsteps.
My interest in fenretinide started when I read about it in an article published in the prestigious journal “Blood” in late 2026. The abstract of the article is given below and you can read the full text of it for free by clicking on the link provided.
http://www.bloodjournal.org/cgi/content/full/103/9/3516
Blood. 2026 May 1;103(9):3516-20. Epub 2026 Dec 24
Fenretinide enhances rituximab-induced cytotoxicity against B-cell lymphoma xenografts through a caspase-dependent mechanism
Gopal AK, Pagel JM, Hedin N, Press OW.
Seattle Cancer Care Alliance, Mailstop G6-800, Rm 6802, University of Washington, 825 Eastlake Ave E, Seattle, 98109
The anti-CD20 monoclonal antibody rituximab induces remission in 40% to 60% of patients with indolent B-cell lymphoma, but virtually all patients have relapses. We evaluated the efficacy of concurrent administration of another biologic agent, N-(4-hydroxyphenyl) retinamide (4HPR, fenretinide) with rituximab against a variety of human B-cell lymphoma cell lines (Ramos, DHL-4, and FL-18) in vivo. Concurrent 4HPR and rituximab administration prevented tumor progression of lymphoma-bearing mice in a minimal disease model (rituximab + 4HPR, 100% progression free; rituximab alone, 37.5% progression free, P =.01; 4HPR alone, 12.5% progression free, P <.01; controls, 0% progression free, P <.01). Combinations of 4HPR + rituximab exceeded the predicted 50% additive rate of disease control from each agent alone (P =.038). Administering 4HPR and rituximab to mice with established tumors induced complete responses (CRs) in 80% of animals compared with 20% to 40% CRs using either agent alone (P =.07), resulting in significantly improved survival. Tumors harvested from 4HPR + rituximab-treated mice displayed elevated caspase activation compared with untreated controls (P =.02). Adding a broad-spectrum caspase inhibitor in vivo fully abrogated the antitumor effects of 4HPR + rituximab (P =.05). These results establish the efficacy of 4HPR/rituximab combinations, confirm their caspase-mediated mechanism of action, and offer the potential for disease control with minimal toxicity for patients with B-cell malignancies.
PMID: 14695237
____________
As the abstract points out, this was a mouse study. Several types of B-cell cancers were studied. In mice with only minimum residual disease, the combination of Rituxan + Fenretinide caused prevention of disease progression in 100% of the mice, compared to 37.5% for mice treated with Rituxan alone. Equally important, when the combination was given to mice with established tumors, 80% of the mice got complete responses, compared to less than half that got equivalent responses when either of the two drugs was given by itself. Pretty impressive! Pictures are a lot easier to understand than words. Here are a few of the graphs from this paper that caught my attention.
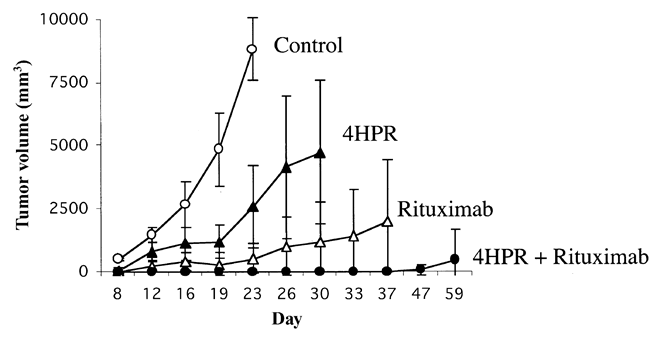
Tumor Progression with fenretinide (4HPR) and Rituxan - MRD
Blood. 2026 May 1;103(9):3516-20.
In this experiment, all of the mice had only minimal residual disease to begin with. None of the 8 mice treated with fenretinide and Rituxan combination developed palpable tumors during treatment. As you can see in the graph above, these lucky mice had next to zero tumor volume 47 days out! Compare this with 5/8 (63%) of mice treated with Rituxan alone that developed tumors, and 7/8 (88%) of mice treated with fenretinide alone, and you can understand my excitement. The effects of fenretinide and Rituxan appear to be more than just a sum of the two drugs, in this in vivo experiment.
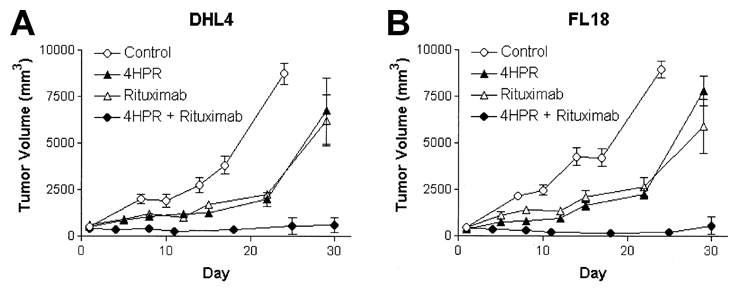
Tumor Progression with fenretinide (4HPR) and Rituxan - Palpable Tumors
Blood. 2026 May 1;103(9):3516-20.
In this experiment, mice were inoculated with cancer cells (two types of cancer cells, DHL4 and FL18) and left to develop palpable tumors. They were then divided into 4 groups, and treated with R + fenretinide, or each drug by itself, or none. Once again, the mice that got both the drugs did far better than the ones that got either Rituxan or fenretinide as single agents.
True, these are mouse studies, and there is a big leap in going from mouse to man. However, this is not as much of a leap in this particular situation. We know that Rituxan works in CLL, just not as well as we would wish. Fenretinide has shown itself to be a potent anti-cancer drug in many different types of cancer. Both drugs have well established toxicity profiles in human beings. The mouse study suggest there may be synergy here. Will there be synergy of the two drugs, when given to CLL patients? Aha! That is the million dollar question — and that is why this clinical trial is important.
For those of you interested in the mechanism(s) by which fenretinide works, this very recent paper should be a good starting point.
Apoptosis. 2026 Oct;11(10):1677-94
Mechanisms of fenretinide-induced apoptosis
Hail N Jr, Kim HJ, Lotan R.
Department of Clinical Pharmacy, School of Pharmacy, The University of Colorado at Denver and Health Sciences Center, Box C238, 80262
Fenretinide, a synthetic retinoid, has emerged as a promising anticancer agent based on numerous in vitro and animal studies, as well as chemoprevention clinical trials. In vitro observations suggest that the anticancer activity of fenretinide may arise from its ability to induce apoptosis in tumor cells. Diverse signaling molecules including reactive oxygen species, ceramide, and ganglioside GD3 can mediate apoptosis induction by fenretinide in transformed, premalignant, and malignant cells. In many cell types, these signaling intermediates appear to be induced by mechanisms that are independent of retinoic acid receptor activation, and ultimately initiate the intrinsic or mitochondrial-mediated pathway of cell elimination. Numerous investigations conducted during the past 10 years have discovered a great deal about the apoptogenic activity of fenretinide. In this review we explore the mechanisms associated with fenretinide-induced apoptosis and highlight certain mechanistic underpinnings of fenretinide-induced cell death that remain poorly understood and thus warrant further characterization.
PMID: 16850162
____________
As mentioned above, fenretinide has been actively studied in cancer research for many years. One of the major studies looked at long term use of fenretinide for the prevention of breast cancer (the abstract is below and you can read the full article by clicking on the link provided). This was a large study involving close to 3,000 patients, roughly half of whom were treated with fenretinide for 5 years, while the other half served as the control group. Both the size of the study and the long duration makes the results very robust. This is just one example of several large and long term studies that have scrutinized safety of this drug in human beings.
As we discussed above, fenretinide is a synthetic analog of vitamin A. It is believed that when patients are given fenretinide, it competes with vitamin A for the same receptors in the eye. Now it is well known that vitamin A is necessary for night vision. In fact, poor vision under low light conditions is a major health problem in many parts of the world where there is a chronic deficiency of vitamin A in the diet, a condition known as night-blindness or nyctalopia. It is reasonable to expect that when there is a lot of fenretinide in the body competing for the same receptors that would normally be targeted by vitamin A, there may be reduced night vision. Indeed, this was one of the most common adverse effects seen in the breast cancer study, along with dermatological disorders. The good news is that the effect is seen to be temporary and the adverse effects gradually fade away when fenretinide administration is stopped. Moral of the story: if you are going to indulge in significant amounts of fenretinide, be sure you are not the designated driver after a night out on the town — even if you don't drink. This is a restriction I could live with, especially since I don’t like driving at night anyway.
Fenretinide is a fat soluble compound, as is vitamin A. For that reason, its absorption is facilitated by fat in the diet. I understand the capsules used in the trial use corn oil as the medium, with a specified amount of fenretinide dissolved in it. Patients are also counseled to take the capsules along with a meal with a reasonable amount of fat and/or protein in it, such as a glass of whole milk, for example. People who are on high fat / low carb diets such as the Atkin’s diet should do just fine! For others not used to a high-fat diet, it may lead to some of the gastrointestinal symptoms. May I suggest that you increase your exercise level during this period to compensate for the extra calories, so that you keep your svelte and youthful figure? (The protocol appendix provides patients with healthy alternatives to whole milk.)
Article from JCO (Full-text PDF)
J Clin Oncol. 2026 Mar 15;19(6):1664-70.
Safety of the synthetic retinoid fenretinide: long-term results from a controlled clinical trial for the prevention of contralateral breast cancer.
Camerini T, Mariani L, De Palo G, Marubini E, Di Mauro MG, Decensi A, Costa A, Veronesi U.
Istituto Nazionale Tumori, Milan, Italy.
PURPOSE: To describe the pattern of occurrence of adverse events commonly arising during treatment with fenretinide, a synthetic retinoid under investigation for cancer prevention.
PATIENTS AND METHODS: The series includes 2,867 women accrued in a trial aimed at assessing the effect of fenretinide on the prevention of second breast malignancy. Women were randomly assigned to receive no treatment (1,435 patients) or 5-year fenretinide treatment (1,432 patients). In terms of disease recurrence in the breast, the trial showed a possible beneficial effect of the compound in premenopausal women, and an opposite trend in postmenopausal women. End points considered for safety assessment were the occurrence of diminished dark adaptation, dermatologic disorders, gastrointestinal symptoms, disorders of the ocular surface, and abnormal laboratory values.
RESULTS: The most common adverse events were diminished dark adaptation (cumulative incidence, 19.0%) and dermatologic disorders (18.6%). Less common events were gastrointestinal symptoms (13.0%) and disorders of the ocular surface (10.9%). In comparison, incidence figures in the control arm were 2.9% for diminished dark adaptation, 2.9% for dermatologic disorders, 5.4% for gastrointestinal symptoms, and 3.2% for disorders of the ocular surface. Symptoms occurring during fenretinide treatment tended to recover with time. No between-group difference was observed for the occurrence of laboratory data abnormalities. Overall, 63 (4.4%) treatment discontinuations were caused by adverse events.
CONCLUSION: Given the number of patients involved in the study and the prolonged intake of the drug, the experience on fenretinide tolerability can be considered sufficiently reassuring to justify further testing of the retinoid.
PMID: 11250995
____________

This is a Phase-2 clinical trial, recruiting patients with B-cell cancers (CLL patients as well as folks with non-Hodgkin’s lymphoma, mantle cell lymphoma, etc.) with measurable disease. The inclusion criteria are a breeze! Oh yes, the usual bit about pregnant and breast-feeding women and HIV positive patients being excluded. Other than that, you have to be an adult, able to give informed consent, and not absolutely on your last legs in this world. Piece of cake!
The Phase-1 part of the trial (just concluded) used single agent fenretinide only, with dose escalation between 700-900 mg/m2 given twice a day for 5 days out of each week. The point in doing these dose escalation studies is to fix the level of the drug that can be used safely in the next phase of the trial. That first part is now done and the researchers are moving on to the second phase of the trial — the interesting part from my perspective.
In this Phase-2 study, once again patients will be given fenretinide as capsules, to be taken twice a day for five days out of every week. Starting in week five, Rituxan will be given as an infusion at the so-called standard dose of 375 mg/m2 (see: Rituxan Shaving), once a week for 4 weeks. Patients will continue taking fenretinide as before all through the 8 weeks. At the end of the 8 weeks, for patients who show no disease progression or excessive adverse effects, the clinical trial allows them to continue taking fenretinide as before. These patients will also get additional Rituxan infusions once every three months. Pay attention to the units of the drug administered: milligrams/meter squared. In other words, the amount of drug any given patient will get depends on his/her height and weight, which in turn determines the BSA (Body Surface Area). Most adults have a BSA of between 2.0 - 2.5 square meters. For example, if your BSA is a slender 2.0, you will get 375 X 2.0 = 750 mg of Rituxan each time. If you want to know how to calculate your BSA, here is a helpful link: BSA Calculation.
The consent form for patients who wish to participate in this trial can be viewed and downloaded as a pdf through this link: Consent Form. It also has contact information, phone numbers and a whole lot of other stuff, all 12 pages of it. I urge you to read it carefully, if you are interested in this clinical trial. Unfortunately, since this clinical trial protocol includes a range of blood cancers, the guidance is quite general and not specific for CLL patients. NHL folks, for example, don’t have any significant percent of their cancer in the blood and for these guys the only way of monitoring the status of the disease is via CT scans, PET scans, lymph node biopsies, etc. CLL is a little easier to track, since it is a leukemia and therefore shows its presence in the peripheral blood. CT scans and PET scans may not be necessary for our guys.
The only way you can be really sure about the details as they pertain to you is to call and ask the questions. Don’t be shy about it! Volunteering to participate in clinical trials is a big deal and it is very important that you get your questions answered. The appropriate first point of contact would be the trial co-ordinator, Jennifer (Jen) Roden, whose contact information is given below. She will, of course, pass along to Professor Gopal any questions she cannot answer.
Jennifer E. Roden
Clinical Research Coordinator
University of Washington
Seattle Cancer Care Alliance
825 Eastlake Ave. E/MS G6-800
Seattle, WA 98109
Phone: 206-288-6721 Fax: 206-288-1130

Dr. Ajay Gopal (here is a link to his curriculum vitae), the Principal Investigator conducting this trial, is an approachable and likable guy. I have found him to be candid and responsive over the past year, as we worked out the details of our sponsorship of this Phase-2 clinical trial. I think you will find him helpful in answering your questions. If you call him, do be sure and tell him CLL Topics sent you and that I am keeping my fingers, toes and eyes crossed for the success of his clinical trial.
 Enter Keywords: |
———
Disclaimer: The content of this website is intended for information only and is NOT meant to be medical advice. Please be sure to consult and follow the advice of your doctors on all medical matters.
Copyright Notice:
Copyright © 2026-2007 CLL Topics, Inc. All Rights Reserved.
All materials contained on this site are protected by United States copyright law and may not be reproduced, distributed, transmitted, displayed, published or broadcast without the prior written permission of CLL Topics, Inc. You may not alter or remove any trademark, copyright or other notice from copies of the content.
However, you may download and print material from CLLTopics.org exclusively for your personal, noncommercial use.
———
